I recently started using a 50-year-old vacuum-seal flask that belonged to my Grandpa so that I don’t have to leave the dungeon as often to procure more caffeine. Besides looking totally awesome on my side desk, this thing still works like new, at least as far as I can tell — it’s older than I am.

Of course this got me to wondering how exactly vacuum-seal flasks, better known in household circles as Thermoses work, and how they were invented. The vacuum-seal flask is surprisingly old technology. It was first invented by Scottish chemist Sir James Dewar and presented to the Royal Institute in 1892. Six years later, he would be the first person to liquefy hydrogen and is considered a founding father of cryogenics.
At the time, liquefying gases was an expensive process, and it was important to keep them in a fluid state as long as possible. Sir Dewar’s flask consisted of two flasks separated by a near-vacuum that does much of the job of heat retention. Heat cannot travel through a vacuum, so the contents stay at-temperature for much longer than they would outside the flask.
These vessels have a ton of uses other than keeping your coffee hot or your tea iced. They are widely used throughout the industrial, scientific, and medical fields for everything from preserving bodily fluids and tissue to measuring electric power, recording the weather, and detecting an airplane’s rate of climb. Vacuum-seal flasks are also used in MRI machines to keep the superconducting magnet cool. And they’re still used to keep liquefied gases liquid.
Highways for Heat
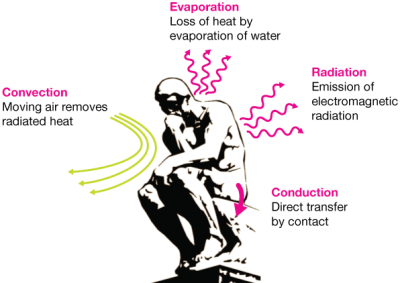
Why are vacuum-seal flasks so effective at heat retention? That depends on how you look at it, as there are four major ways to describe heat loss — convection, conduction, radiation, and evaporation.
Starting from the top, that tight-fitting lid seals off the chamber and prevents heat transfer by both convection and evaporation. The near-vacuum between the inner and outer flasks helps to further prevent heat loss by convection.
The thin walls of the flask prevent heat conduction the same way you can put a single unfolded sheet of aluminum foil in the toaster oven, set it as high as you want, and pull it out bare-handed when your bratwurst bun is toasted. Thin walls have less mass, so they don’t conduct heat away from the liquid inside the vessel.
Back when the flasks were lined with glass, they had a silver coating that prevented heat lost by radiation. People got tired of replacing their Thermos every time they dropped it, so today’s flasks are much more likely to be lined with steel instead.
Missed Opportunities
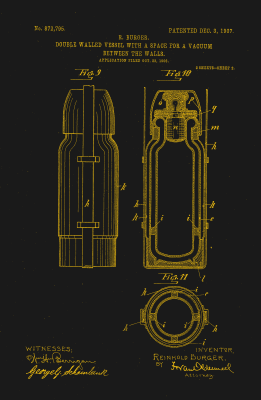
Looking back from the 21st century, it’s easy to assume that both hot and cool applications were thought of at the same time, but that’s not the case. The vacuum-seal flask was not Sir James Dewar’s first foray into long-term temperature maintenance. Along with physicist and fellow countryman Peter Tait, he had developed a vacuum-insulated goblet to keep substances warm some twenty years earlier. Sir Dewar’s vacuum-seal flask intended for keeping liquefied gases cool had a much narrower neck than the hot goblet, and it was jacketed in a silver coating that prevented heat loss by radiation.
Sir Dewar was famously hot-headed. He’d had a falling out with Alfred Nobel over the patent for cordite, and never did patent his vacuum-seal flask. One of his glassblowers, Reinhold Burger along with another glassblower Albert Aschenbrenner discovered that the flask also kept baby milk warm for several hours, and realized its commercial potential. They patented the Thermos in 1904. Sir Dewar later lost his court case against the company. Although they recognized him as the inventor, he had not patented his invention, so they were within their rights.
Here’s a fun fact about thermodynamics, courtesy of our own [Adam Zeloof] — a beer on its side in the fridge will cool faster than the vertical can next to it. This is because horizontal cylinders have higher heat transfer than vertical cylinders as far as natural convection is concerned. That goes for any liquid in a sealed cylinder, not just the fizzy, alcoholic kind. So, remember that the next time the bodega only has warm cases of Pabst Blue Ribbon and bottles of Club Mate. Cool it down sideways, and then pour it into a vacuum-seal flask.















Don’t forget to fill (and empty) your vacuum-sealed flask with hot or cold water first to eliminate its thermal capacity before filling with fluid of choice!
I’ve never thought about that, but it makes perfect sense. Then again, I’m the kind of person that thinks my coffee stays too hot for too long in a thermos.
So pre-chill a thermos (lid off) in the fridge before filling it with coffee. Heating up the inside of the flask will take some of the heat out.
Yeah no I wouldn’t do that. Had a thermos burst like that. Produced quite a loud bang, and a shame of the coffee. (Thermos didn’t even come out of the fridge, but out of a cold cupboard.)
For cold drinks, fill it with ice, then the rest with water. A good thermos will still have ice water 2-3 days later.
For longer hot time, keep the thermos upside down.
And yes, I did test this , and the difference is significant (A-B controlled test when I bought two identical new thermoses).
Here’s another trick I’ve learned: don’t store the actual beverage in the flask; store excess hot water for brewing fresh, instead. Hot water doesn’t spoil or turn sour over time like coffee does. ;)
They also can be made more compact than an equivalent insulator. Whenever I see a mug that is vacuum and thick, it reminds me of when companies put weights into plastic widgets to make them seem more valuable.
(referring to Title Photo)
THAT’S a dungeon????
B^)
It’s a mid-century dungeon.
B^)
Of course..
Do you not see the ominous comfy chair….
Now THIS is a thermos!
https://ntrs.nasa.gov/api/citations/19730017162/downloads/19730017162.pdf
FYI, if you want to use a shorter form than the full “Sir James Dewar”, it’s “Sir James”, or “Dewar”.
” Although they recognized him as the inventor, he had not patented his invention, so they were within their rights.”
Much in the universe is explained. ;-)
I thought that was extremely telling as well. What douchebags.
“People got tired of replacing their Thermos every time they dropped it, so today’s flasks are much more likely to be lined with steel instead.”
Back then, any hardware store “worth its salt” would have a replacement inner flask somewhere on the shelves.
Although, not all Thermos[tm] (Thermii? B^) had replaceable flasks.
OT: there was a “Colombo” episode, guest starring Johnny Cash, where a missing Thermos was a plot point.
1974 Columbo season 3 episode 4 is “Swan Song” which had Johnny Cash as the guest stars.
Thanks!
Lived in China for a while. Everyone drinks tea, right? We had a central water boiler in the office, and everyone had a giant (3 L?) thermos bottle that was essentially a metal cage around a visible glass vacuum vessel. They broke, but when you did, they only cost like $0.35 to get a new one, and we had a store of those by the boiler.
Other tricks? Make sure it’s full of cold air, fill it with boiling water but only halfway. Cork it. In a minute, either the cork goes flying to the ceiling with a loud pop, or you’re cleaning up the mess and out $0.35.
“Heat cannot travel through a vacuum” well, it can as infrared radiation (as you pointed out lower down, the silvering doesn’t prevent this though, but it does reduce it a lot).
doesnt nasa use gold to reflect IR? probably a bit on the expensive side for hot coffee though.
If you are talking about the golden visors in the space suits, AFAIK, that’s to filter ultraviolet.
no, talking about the gold foil they use around satellites, etc
Got to add my Thermos joke here… a football player goes to training & sees the coach with a flask. He’s fascinated as the coach explains that whilst today it’s keeping his coffee nice & hot, he can also use it to keep things cold. “I’ll buy one at the weekend!” he tells the coach.
Next week he arrives for training with his brand-new flask “hey!” says the coach “I see you bought a flask, what do you have in it?” The football player replies “tomato soup and a chocolate ice-cream”
There used to be an old commercial for Thermos where the speaker would say, “It keeps hot things hot, and cold things cold, how it knows what’s in there I just don’t know.”
I’ll add a funny movie reference: one of the guards in the movie “Hudson Hawk” was pouring his lunch (spaghetti and a tomato-based sauce) from a thermos designed for liquids. (they did make one designed for more solid foods with a wider throat)
I grew up carrying a glass Thermos to school. Suspending a 1/2 lb of hot soup by the walls of a thin wall glass vacuum flask and handing it into the care of a small child sells a lot of replacement liners.
Now I carry my steel water flask. Though Iʻve found that the metal is far tougher, itʻs also far more thermally conductive and the lids donʻt seem to have any insulation like the Thermos bottles. Here in Hawaiʻiʻs humid climate, they condense copiously.
I got a stainless steel food thermos, which i tested out against grandmas old, still working, glass thermos with boiling water. Against all expectations, the stainless one won. So clearly, caveat emptor applies to thermoses as well.
> These vessels have a ton of uses other than keeping your coffee hot or your tea iced.
If it’s all the same, I’d rather keep my tea hot and my coffee iced…
No, that is unacceptable, leave now, before we make you leave!
B^)
Seeing that flask brings back memories.
I love the way they use to decorate these things. Nowadays it is just logos.
I still have my all stainless steel Thermos from 30+ years ago when working as a Burner ( Oxy/ acetylene torch ) for General Dynamics in Quincy Massachusetts.
That thing had fallen of the top deck of a ship in dry dock ( about ten stories high ) ,only thing that happened to it was a small dent on the bottom and the handle that was designed to be replaceable cracked ( fixed that with two hose clamps and the handle from a metal trash can).
Pretty sure the rate of heat conduction would be proportional to the cross sectional area, not the mass. The amount of energy it would take to heat the material by one degree would be proportional to its mass.
Sir James was a subject of a clerihew, a poetic form created (and, in this case, written) by his contemporary, Edmund Clerihew Bentley.
Professor Dewar
is a better man that you are.
None of you asses
can condense gases.
I use a thermos to carry water during the winter when it reaches -35 dec Celsius. The water won’t freeze throughout the whole 12 hours work shift, but I avoid puting any carbohydrated solution in it since it reaches around 30 deg C, which is an optimal temperature for bacteria cultures.
These flasks are the reason why I never believed that you will insta-freeze in space without a suit.
Dewar…hardly knew her.
What’s the reason for the sounds from thermos flask when it’s in working condition and why not if it’s damaged