In the technologically-underpinned modern world, most of us interact with a battery of some sort every day. Whether that’s the starter battery in a car, the lithium battery in a phone, or even just the coin cell battery in a wrist watch, batteries underpin a lot of what makes society possible now. Not so in the early 1800s when chemists and physicists were first building and experimenting with batteries. And those batteries were enormous, non-rechargable, and fairly fragile to boot. Not something suited for powering much of anything, but if you want to explore what it would have been like to use one of these devices, follow along with [Christopher]’s build of a voltaic pile.
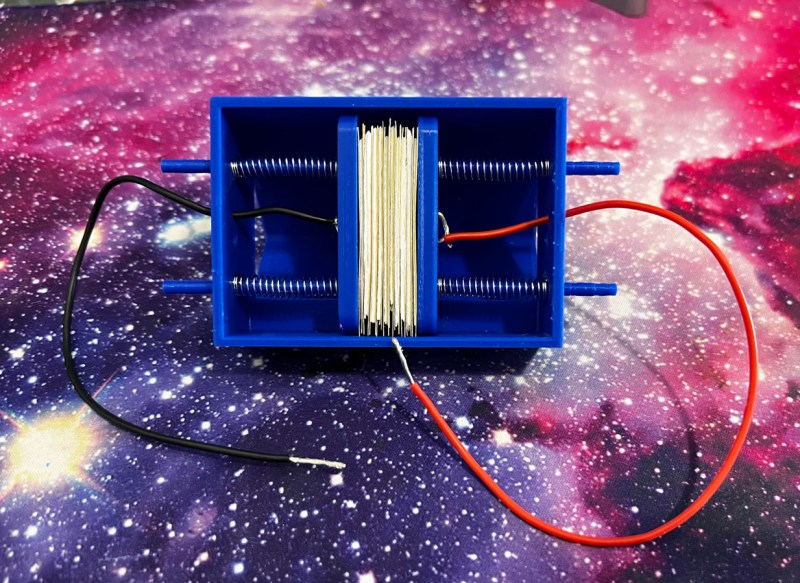
The voltaic pile is historically constructed using discs of alternating zinc and copper paired with an acidic electrolyte, but this build uses much more convenient metallic strips instead of larger discs. Tissues are used to facilitate absorption of the electrolyte solution, and a 3D-printed case is used to help hold everything together, with a spring mechanism built-in which keeps pressure on the alternating metallic strips. The electrolyte is nothing more than salt water here, which transports the ions from one end of the battery through the circuit to the other. With everything assembled in the 3D printed case, the voltaic pile creates almost 3 volts, although [Christopher] notes it should be making closer to 5 volts but there’s likely an internal short somewhere.
While voltaic piles don’t have much use anymore due to their limitations, [Christopher] intended this build to be used more of an educational demonstration than a practical application. It’s much easier to build this one than a more historically accurate one as well, and the use of springs and 3D printed parts means that it could be made to have a larger or smaller voltage by simply adding or removing cells within the pile. It’s also similar to the lemon or potato battery, the latter of which we’ve actually seen put to practical use in this 12V potato battery pack.















That is a great project to try with the kids, but they’d probably then want to move on to something rechargeable.
Good for schools too, and doesn’t go bad like potatoes.
I wonder; suppose you’re at home when the power goes out and you want to recharge your phone, but all you have is a bunch of kitchen supplies like aluminum foil, salt, tissue paper…
Could you construct a voltaic pile powerful enough to do it? No cheating like running off to the hardware store – just whatever is in a typical kitchen.
Or make a peltier generator out of fence wire (or nichrome toaster wire) and copper. Easy to get a couple of millivolts per junction pair, at an amp or more. Put a few thousand in series on a gas flame or a candle. Good for a watt or two. This I have tried. It works, but quite a chore, and you must use small copper wire and large iron wire: minimize the heat conduction through the copper, and minimize the ohmic loss in the iron. I used 12 ga. fence wire and 24 ga. telephone wire.
But for a voltaic battery: copper and aluminum are about 1.8 volts apart on the electrochemical series. If you could keep the aluminum from oxidizing, it might work. Lye as an electrolyte, maybe. Pennies and aluminum foil? Never tried this. Don’t have pennies around here anyway.
I’d expect lye to quickly dissolve aluminum foil.
Pennies are no made out of copper anymore. They havent been for a long time so you would need really old ones
If we look at the question practically then it is much easier to use a stepper motor from a printer as a generator. It is very hard to build anything without having electricity to do the soldering though.
You can solder without electricity. An electrical soldering iron is very convenient, but people have been soldering things for 5000 years.
https://en.wikipedia.org/wiki/Soldering
That’s exactly the point though. It’s very hard to build -anything- on the spot if all you have is random everyday stuff from your house. Building a generator out of a stepper motor with things you just happen to have laying around is a difficult task, because you also need to fashion the mechanics and the voltage regulator somehow, and then you have to keep cranking it for an hour to actually charge the battery.
A voltaic pile is easier in a sense that you can just stack things together until you have approximately 5 Volts and then hook it up to a cut USB cable. The hard part is figuring out what to use.
Aluminium foil, salt, water, and crushed carbon tablets makes a rudimentary battery.
Using a motor as generator would be much more efficient, but there’s still the problem of what’s driving the generator. Don’t look at me, I’m busy boarding up the windows in case the zombies attack.
For soldering I bet many Hack-a-Day’ers have butane-powered soldering irons. I have a Weller PSI100K for when I don’t have an outlet for my soldering station or I’m lazy.
The recording is awesome to visualize nothing. Specially with this background.