By far, the most widely used psychoactive substance in the world is caffeine. It’s farmed around the world in virtually every place that it has cropped up, most commonly on coffee plants, tea plants, and cocoa plants. But is also found in other less common plants like the yaupon holly in the southeastern United States and yerba maté holly in South America. For how common it is and how long humans have been consuming it, it’s always been a bit difficult to quantify exactly how much is in any given beverage, but [Johnowhitaker] has a solution to that.
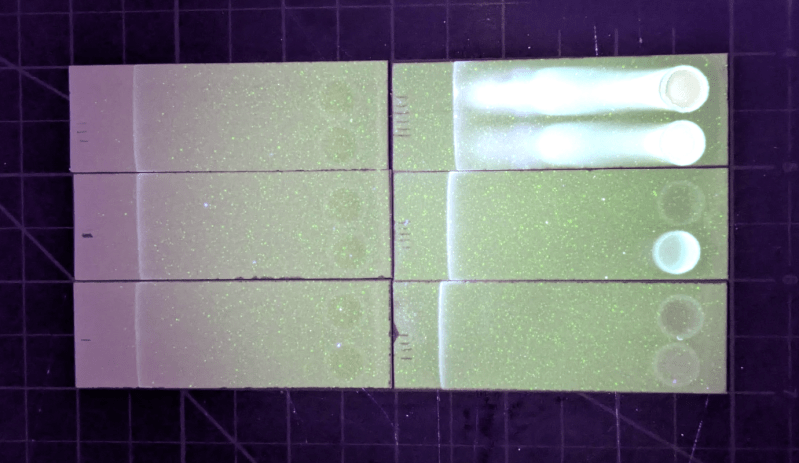
This build uses a practice called thin layer chromatography, which separates the components of a mixture by allowing them to travel at different rates across a thin adsorbent layer using a solvent. Different components will move to different places allowing them to be individually measured. In this case, the solvent is ethyl acetate and when the samples of various beverages are exposed to it on a thin strip, the caffeine will move to a predictable location and will show up as a dark smudge under UV light. The smudge’s dimensions can then be accurately measured to indicate the caffeine quantity, and compared against known reference samples.
Although this build does require a few specialized compounds and equipment, it’s by far a simpler and less expensive way of figuring out how much caffeine is in a product than other methods like high-performance liquid chromatography or gas chromatography, both of which can require extremely expensive setups. Plus [Johnowhitaker]’s results all match the pure samples as well as the amounts reported in various beverages so he’s pretty confident in his experimental results on beverages which haven’t provided that information directly.
If you need a sample for your own lab, we covered a method on how to make pure caffeine at home a while back.















I guess that is the only way to get that 126 Octane coffee.
Be buzzing like a neon power supply.
If your coffee is that desperate to detonate you are doing something wrong.
“CAFFEINE” being the lawful alkaloid that works with TLC just like all the other ones do, and this is why caffeine is often included as a standard reference or positive control in TLC analyses of seized drugs.
Huh neat, TIL!
Awesome work!! Many people myself included suffer from a caffeine sensitivity which generates cardiac arrythmias = nasty. But you’re never sure whether you’ve been given decaf or regular despite asking for it. At least you could take a sample home and test it, unless maybe you whip out your ethyl acetate TLC kit at the restaurant table and run a quick chemistry lab…
Eh…if you are paying other people to make your coffee for you you should expect it to be made correctly.
And if you are drinking cheap/bad/decaf coffee, you might as well just carry around a jar of instant decaf and order a mug of hot water and sugar/creamer/etc so you can be sure it’s decaf.
I honestly don’t understand how so many places can serve bad coffee and everyone just tolerates it.
Making good coffee is TRIVIALLY EASY.
Use the CORRECT temperature water.
Add coffee to water, or water to coffee.
Let it sit if that’s how your brewing method works. I’m not your mom. Use whatever method you like as long as it doesn’t break rules 1 or 4.
DO NOT KEEP HEATING IT.
Done.
Unless you have criminally bad coffee, it will be fine.
And I mean that. Use the cheap stuff from a big food-service can like Folgers or Maxwell House and the CORRECT temperature water and it will be better than that burnt $9 Dunkin swill or $12 Starbucks (gag) garbage.
It’s not going to win any awards, but even those vacuum packed bricks of super cheap coffee become okay.(Not GOOD but surprisingly drinkable.)
I keep saying CORRECT temperature.
104-106F
Yes. It IS that small of a window.
Yes, you will have to adjust for STP because it is such a small window.
(Sorry I’m not breaking out the decimals to put it in Celsius. You can do the unit conversion yourself.)
103 is debatable. Some like it, I think it barely tastes like coffee.
107 is too hot. You start getting all the sour and bitter nasties.
Coffee uses chemistry set rules.
X compound you want starts dissolving at Y temp.
J compound you don’t want starts dissolving at Q temp.
(Yes everything is always reacting but there are typically sharp delineations.)
And after it brews DON’T KEEP HEATING IT.
Coffee oxidizes. Chemicals break down into oher nasty tasting ones.
You can reheat it. You don’t NEED to drink it cold.
But don’t you DARE put it on a burner or hot plate to keep it near/at boiling.
Just let it come down to room temp and leave it till you want it.
It will even be fine overnight if you cover it. Just reheat a mug tomorrow morning if you are feeling lazy. It will be like 90% as good. Heck maybe you even like it better the next day.
I realize it probably feels like there is more to it.
There really isn’t.
Part of the reason I typed all this is so you didn’t immediately dismiss it as too simple and therefore improbable.
104-106F water + coffee.
If you aren’t doing it this way and you think you like coffee… Try it.
If you like it, you can get an electric kettle with temperature control super cheap nowadays.
A french press is also cheap, and probably the easiest way to do it.
Start water. Clean yesterday’s grounds. Rinse out press. Dump in new grounds (you don’t even need special grounds. Use regular ground coffee and just avoid drinking the VERY LAST sip from that cup unless you like the hick sludge.)
Water should be ready. NOT BOILING. Dump it in. Come back in a few minutes and press it down.
If you have a kettle with no temperature control you can let it boil, then put a thermometer in it and WAIT until it falls back down to the right temp. Heck you can do this method in a pot of you want.
104-106F