McDonald’s is serious about their fries. When they were forced by shifting public opinion (drunkenly swaggering around as it always does) to switch from their beef tallow and cottonseed oil mixture to a vegetable oil mixture; they spent millions to find a solution that retained the taste. How they make the fries is not the worlds most closely guarded secret, but they do have a unique flavor, texture, and appearance which is a product of lots of large scale industrial processes. [J. Kenji López-Alt] decided to reverse engineer the process.
His first problem was of procurement. He could easily buy cooked fries, but he needed the frozen fries from McDonald’s to begin his reverse engineering. McDonald’s refused to sell him uncooked fries, “They just don’t do that,” one employee informed him. He reached out to his audience, and one of them had access to a charlatan. The mountebank made quick work of the McDonald’s employees and soon [J. Kenji] had a few bags of the frozen potato slivers to work with.
What follows next was both entertaining and informative. At one point he actually brought out a Starrett dial caliper to measure the fries; they were 0.25in squares in cross section. Lots of research and experimentation was done to get that texture. For example, McDonald’s fries aren’t just frozen raw potatoes. They are, in fact; blanched, flash fried, frozen and then fried again. Getting this process right was a challenge, but he arrived at similar fries by employing his sous vide cooker.
He then wanted to see if he could come up with a french fry recipe that not only allowed the home chef to make their own McDonald’s fries, but improve on them as well. It gets into some food chemistry here. For example he found that the same effect as blanching could be produced by boiling the fries; if you added vinegar to keep the cell walls from disintegrating.
The article certainly shows how knowledge of the chemistry behind cooking can improve the results.




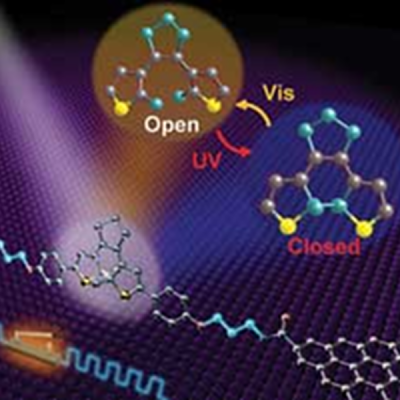 Placing electrodes on the molecule interferes with the process. Depending on the kind of electrode, the switch will get stuck in the on or off position. Researchers at Peking University in Beijing determined that
Placing electrodes on the molecule interferes with the process. Depending on the kind of electrode, the switch will get stuck in the on or off position. Researchers at Peking University in Beijing determined that