When it comes to the history of medicine and drugs, Aspirin, or more properly acetyl-salicylic acid, is one of the more interesting stories. Plants rich in salicalates were used as medicines more than four thousand years ago, and in the fourth century BC, [Hippocrates] noted a powder made from willow bark was an excellent analgesic. It was only in the 1800s that acetylated salicylic acid was first synthesized. In 1897, chemists at Bayer gave this ancient remedy a new name: Aspirin. It’s on the WHO List of Essential Medicines, but somehow millions of people don’t have access to this pill found in every pharmacy.
[M. Bindhammer] is working to make Aspirin for Everyone for his entry to the Hackaday Prize, using a small portable lab designed around chemicals that can be easily obtained.
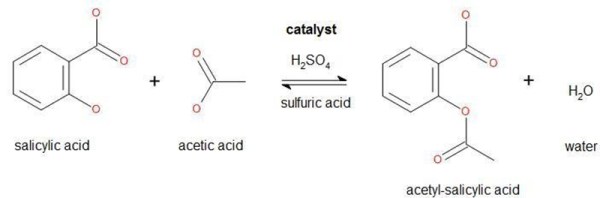
The most common synthesis of Aspirin is salicylic acid treated with acetic anhydrate. Acetic anhydrate is used for the synthesis of heroin, and of course the availability of this heavily restricted by the DEA. Instead, [M. Bindhammer] will use a different method using salicylic acid and acetic acid. If you’re keeping track, that’s replacing a chemical on a DEA list of precursors with very strong vinegar.
[M. Bindhammer] even has a design for the lab that will produce the Aspirin, and it’s small enough to fit in a very large pocket. Everything that is needed for the production of acetyl-salicylic acid is there, including a reaction vessel with a heating element, a water/oil bath, flask, an Allihn condenser, and a vacuum filtering flask. Even if shipping millions of pills to far-flung reaches of the planet were easy, it’s still an exceptional Hackaday Prize entry.