Engineering for medical, automotive, and aerospace is highly regulated. It’s not difficult to see why: lives are often at stake when devices in these fields fail. The cost of certifying and working within established regulations is not insignificant and this is likely the main reason we don’t see a lot of work on Open Hardware in these areas.
Ashwin K. Whitchurch wants to change this and see the introduction of simple but important Open Source medical devices for those who will benefit the most from them. His talk at the Hackaday Superconference explores the possible benefits of Open Medical devices and the challenges that need to be solved for success.
Ashwin discusses a sobering statistic from the World Heath Organization to start off his presentation: about 90% of the world’s investment in medical research benefits only the most affluent 10% of its population. This is likely related to what is known as the 10/90 gap, a finding in 1990 that less than 10% of health investment worldwide was put into developing countries where 90% of preventable deaths occur. This statistic is debated by some, but we think all can agree that applying science and technology to help the sick — no matter their position in life — is a virtue. How can we focus our Open Hardware movement to make advances in medical care available for more people?
We’re delighted that a few of Ashwin’s products which try to address this need were entries in the 2017 Hackaday Prize. His HealthyPi V3 claimed 2nd place and is a patient monitor that records ECG, respiration, pulse-ox, skin temp, and blood pressure. It’s a “hat” for a Raspberry Pi and can be run with or without a screen for the readout. His HeartyPatch project was a Best Product finalist. Based on an ESP8266, it is a wearable single-lead ECG monitor.
These two are interesting products to compare to devices you would find in hospitals in high-income countries. FDA approved patient monitors will cost between $2,000 and $10,000. There are unbranded machines available on markets like AliExpress which cost between $200 and $1,000 but these do not come with certifications and they’re not open source — when they need to be calibrated or repaired what are your options? An ideal Open Source solution would be independently certifiable and calibrated by the care giving institution since proper documentation on doing so would exist. And there is another cost benefit: they can utilize generic consumables, items that can be very expensive if locked into one manufacturer’s brand.
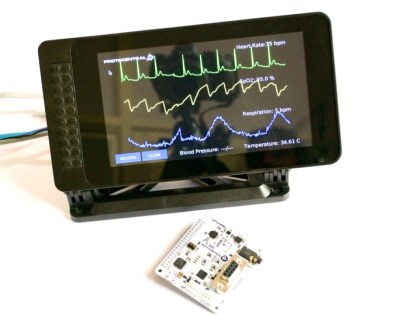
Ashwin mentions that his devices are using the same ICs that are often found in the certified gear. For the patient monitor that’s the AFE4400 for heart rate and pulse oximetry and the ADS1292R multichannel ADC for respiration and ECG. With these silicon solutions available to Open Hardware developers, the concerns for safety and responsible engineering become a matter of established design and verification.
The biggest need for low-cost medical equipment is in places that also have a shortage of specialized medical practitioners. Ashwin envisions low-cost fetal heart monitoring devices for low-income countries where an alarming number of fetal deaths occur during labor. He suggests a device with a user interface simplified for midwife or non-medical birth helpers could do something as simple as indicate that something is normal, or not normal in which case having the mother reposition herself could make the difference.
The challenges here are many, and we’ve moved rather hastily through a lot of the topics Ashwin discusses so make sure you set aside some time to watch his talk. He sees a need for a few things to make Open Source medical devices possible. There must be buy-in from the medical and engineering communities. The products need to be made usable by those without advanced medical degrees and safeguards against misdiagnosis from false positives and negatives need to be addressed. Perhaps the biggest hurdle is to reconcile certification and regulation standards with a new breed of devices not meant to replace what we have, but to fill a need currently not addressed.
If these barriers can be overcome, we will see these devices which are currently developer-grade become consumer-grade and lead to a better quality of care for a large part of the world’s population.















How about getting some better training for the midwife ? If you can get them a costly device, that will need training also ( in the countries he shows, how many midwifes ( for example ) know how to read ? Also, software and labels in the devices would need to be localized. Then said device would need batteris / electrical outlets. And not be stolen. And …..
The guy has good intentions. But many places would benefit more from some low-technological help that is really useful and benefic to them, than just throwing a lot of hi-tech devices that will be hard to mantain.
This is exactly why we need a device is low on tech and low on cost as well, which is what we’re trying to do. The whole point of the project is to look for a device that is less than 50 dollars. Technology should be smart enough to be simple.
Compare it with a doppler which is common in such cases, those would be much more difficult to use. Even starting it up and getting it to work is a challenge.
Healthcare is too lucrative a racket for the medico-underwriting complex to allow anything like this to happen
http://archive.is/gzpng
But how long can that be sustained?
unfortunatelly loooong time, those companies control the legislation … the only way is to simply ignore the big guys … maybe i s a suicide, and not being able to afford the usual expensive way is a death sentence anyway, who will say no, to at least a fighting chance …
What about the current trend makes you think it’s unsustainable? We’re not running out of people to sell healthcare to. They’ll keep happily exploiting people until they are forced to stop, and market pressure will never do that.
Skynet will be addressing that issue.
Can the created AI actually replace the creator?
First, the point of that article was doctor pay. The cost of medical devices (unless you are having a CAT scan) is not the issue.
Second, “single-payer” in the US won’t work UNTIL this issues is resolved.
Third, I appreciated the article link!
Funny how there’s so many reasons it won’t work here even though it does work most everywhere else. I’m beginning to think this is more of an attitude problem.
One of the reasons cellphones with more and better sensors would be a benefit. As dual-use platforms they can bring advantages to a lot of fields from medicine to science. A tricorder for the masses as it where.
… and provide connectivity to get the information quickly to a hospital when necessary. And piggyback on existing infrastructure in a lot of the world.
There are several issues why this can be an problem. First is the platform instability. A simple OS or hardware change (ie, Apple removing phone plug or adopting the lightning connector) can cause lots of headaches, especially to devices already in the field. I worked on a Handspring Visor-based device where the OEM went into a tailspin when the model was discontinued.
Second, in the “West” a device has to have a design history file, and both hardware verification and validations done. Any changes at all require a notification, perhaps amendments to your 510k submission. Anyone who has works with ISO 13485 (GMP) will know intuitively what the issues are, and anyone who has worked under the generic ISO-9000 series will at least have a clue. At least portable (battery) powered devices largely bypass IEC 60601, but are not entirely off the hook if they touch the patient in any way. And just in the last 5 years I’ve seen more and more documentation for bio-compatible materials (a single test to cover the “big three” runs $8000), and that is for a medical lead-wire that “might” touch the patient.
That all said, making low-cost opens source devices for 3d World countries is a good idea. But creating a open source device in the US or EU ios not as easy as it may appear. Now, if they are marketed as a health devices for the consumer market (i.e, like a Fitbit) than your life got easier. And we don’t care about the FDA if selling in Kenya.
Ya can’t sell it in Kenya, can ya?
B^)
Those regulations are there for a reason. I have seen medical devices sold in 3rd world countries which are really bad. What can be brought down is the testing costs. This would actually help in providing more affordable medical devices.
I’ve been wanting to make an open source insulin pump project for a while now. The devices are overpriced, underdeveloped, incompatible, and insurers can artificially limit covered selection and replacement. United Healthcare is guilty of this – they made a deal with Medtronic to be their exclusive vendor for insulin pumps, leaving people like myself that wanted to get t:slim or other pump out in the cold, as well as insuring we don’t have the option of a fully integrated system with accurate CGM since Medtronic does not pair with Dexcom’s much more accurate sensors. Many insurers also *require* your pump to be broken before they will cover a new purchase. The result is crappier care at extortionist cost.
This is the real purpose of excessive regulation in medical hardware. It’s not about safety. Having insurance middle men who effectively write the law is bad for everyone except the insurance companies, who would have thought?
But I thought America had the best health care in the world? The people getting money from the insurance companies keep telling me so…
“about 90% of the world’s investment in medical research benefits only the most affluent 10% of its population”
That is not the explanation of the Global Forum for Health Research, which defines the “10/90 gap” as the approximately 10 percent of total world’s resources devoted to health research which are applied to the health problems of low- and middle-income countries, where approximately 90 percent of the world’s preventable deaths occurred.
Being classed as ‘preventable deaths’ makes it sound like they already have solutions? The disparity doesn’t sound all that bad if these diseases / causes already have solutions, it sounds like the problem is infrastructure either in primary healthcare givers or getting supplies there. A breach birth endangering both mother and child doesn’t really get better with more research, it gets better with access to an operating theater.
My point being, generally that money comes from a different account than the research account. So increasing or changing grant allocation won’t have a gigantic effect on developing regions healthcare.
This project actually received seed funding from the Grand Challenges by the Gates Foundation and other agencies:
https://savinglivesatbirth.net/summaries/2016/473
The term preventable deaths means that these are already being prevented by the use of expensive equipment and expertise that we are use to in developed cities. By making this available to people outside these developed cities is what I meant by preventable deaths.
It’s not always that an operation is the solution. In fact, in most cases it’s as simple as just asking the mother to turn over on the side!
Thanks for mentioning this. The quoted statistic is from the talk slides. I added the “10/90 gap” part into this article and the two may not be from the same studies. I’ve update the article to better reflect this.
91.431% of statistics are made up on the spot!
B^)
There are actually two different versions of this, but both seem to come at the same problem, in different terms.
https://news.harvard.edu/gazette/story/2008/02/medical-basics-still-needed-in-developing-world/ says “about 90 percent of the world’s investment in medical research benefits only the most affluent 10 percent of its population”.
The 10/90 gap is also similar in terms of “It refers to the finding that 90% of worldwide medical research expenditure is targeted at problems affecting only 10% of the world’s population. Applying research results from the rich world to the problems of the poor may be a tempting, potentially easy and convenient solution for this gap.” This would also amount to the same thing ultimately. When we say 10% of the population, it is usually only the top 10% as pointed out about in the Harvard story above.
So where will the open-source designs come from? Will the medical equipment manufacturers that spent millions of dollars developing and qualifying them release them to the public domain?
Or perhaps they will be designed by volunteers in their spare time. But who will evaluate the products and certify that they are adequate?
It would be great if medical stuff would just appear without the high cost, but there are practical issues to deal with. Unless the users are in countries with no laws regulating medical equipment, this is going to be challenging.
I’m getting a nice warm feeling from this. Must be from the plutonium leaking from the pacemaker.
That’s much more likely than trying to sell them in the USA. My father worked for Tektronix for decades. Back in the ’60s, they stopped making medical equipment due to lawsuits. Anytime someone died, the lawyers retained by the survivors sued the makers of every bit of equipment in the room. Even if the manufacturer did nothing wrong, they still had huge legal expenses proving it. Tektronix had a lot more money than a developer of open source hardware, but they decided that it just wasn’t worth it.
Its not just the USA, but even countries like India have become fair ground for such claims. But I do not completely disagree about the risks.
Before actually using devices for diagnosis, some level of reliability is required. Bad signals will equal bad decisions, which result in bad things happening. I consider open source hardware as just a first step towards getting this information out (about what goes into building a device).
The problem here, as I understand it, is that in the US,unlike most other countries The loser in a court case does not have to pay other parties legal costs. So unemployed lawyers and patent trolls drag everyone they can to court, paid out of a percentage of a win, just to try their luck.
I think these patent trolls and lawyers are not on the increase even in India.
I meant on the increase, they’re there everywhere.
The “lawsuit lotto” is a chief driver of US healthcare costs (not the only, but one of the pillars).
For a lot of commonly used medical devices – IV pumps, ECG machines blood pressure. The magic happens not in the machine but in the interpretation of the results or the setting of the parameters.
An infusion pump is just that – an accurate rate controllable pump. What matters is what the physician determines is the correct drug and rate.
An ECG machine is just a visual representation of the electrical signals across the body displayed in a standardized way from standardized electrode placements. It’s up to the cardiologist to determine the significance of that squiggly line.
SPO2 is a bit more complicated as the analysis is done in the machine and the interpretation is displayed. But raw data can be displayed and the physician trained to interpret the results
Blood pressure is a mixture of measured and interpretation. But again a physician could be trained to interpret the results.
But then having the equipment to diagnose is useless with out the equipment to treat. And the resources for recovery.
But then this is all pointless if there is no food, clean water, shelter, law and order or meaningful employment. Just maintaining the lives of the healthy is a big enough job without the challenges of the infirm.
What’s the point in saving someone from a heart attack then send them home to starve to death or drink contaminated water….
It has been my experience (volunteered as a physician in various free clinics around the world/Doctors Without Borders) that medical equipment is not a problem. There is plenty of medical equipment being donated. The problem lies in the following:
(1) Needing someone local on staff to take care of the equipment – maintenance.
(2) Needing someone local on staff that is knowledgeable in its operation and can train other people.
(3) Shipping the actual equipment and easy access to its consumables
Creating new equipment, even if open source, does not deal with this at all. I find engineers that go this route fail to understand that this is a logistics/money/human issue rather one that can be solved by creating new hardware alone. Get the infrastructure in place to solve the above problem and then we can talk about making a new device. People need to have access and knowledge of the basics before going with the latest and greatest.
Thank you. If you’ve been with Doctors without Borders, I would love to have your insight about your experiences.
One of my objectives is to see how this learning curve of training staff and reach can be improved. Training will be required in any case, but can the devices themselves be simple enough that the training can be fast-tracked?
Even in “developed” countries( ok, some would argue Australia is not realy a developed country) anything outside a major city, training and support is difficult and near on impossible. Even just changing batteries can be a difficult task.
I shall agree. I am an engineer in a European medical ventilator manufacturer. Those devices are mainly used by physicians with their default settings… whatever the patient disease. Those are too complex for staff.
My understanding is that medical device are designed by specialists for specialists. Taking into account patient comfort, more and more functioning modes for new markets, more and more subtle parameters or exotic alarms, trying to be “better” than competitors: all is done to rise the complexity of the equipment. It seems to me that regulations and standards are a small issue.
Would it be possible to imagine a device so simple to use that it is safe and reliable but still saves lives ? I guess yes, but needs shall be expressed by the local physicians. May be Open Source for 3rd world is the best way to go.
And as much as mid-level providers, DIYers and the Saturday quarterbacks want to envision that medicine is always easy… I mean, can’t you just google the symptoms?
Look at the state of the art AI failure…
https://www.forbes.com/sites/matthewherper/2017/02/19/md-anderson-benches-ibm-watson-in-setback-for-artificial-intelligence-in-medicine/#22dc40123774
Guess we still need people educated and experienced in medicine.
No, medicine is not easy, but people educated in medicine would also require basic vital sign monitors to take informed decisions.
mythoughts62 says:
(snip)
I knew it! No wonder Tek here went under.
Last I heard they had a buyer show up 2 weeks after they closed their doors forever and scrapped millions worth of kit.
Had they stayed open another two weeks 800+ jobs would have been saved.
Incidentally I invented a working SpO2 sensor for most recent phones.
It uses the previously unused (apart from face detection) front IR emitter and sensor, back camera and LED.
The trick is to get the LED to sync to the IR, thus capturing waveform without ambient light interference.
Just need to do some more work and write an app.
The Samsung Galaxy S had one of these and so do many others, its well worth looking into simply adding a hand selected red LED to phones that do not have LED flash but unpopulated board pad and see what that does.
Do you have this project posted somewhere? Maybe you could put it on Hackaday ! Would be helpful.
On my test S3 there is a lot of A-D range other than on or off.
Have some bare sensors here snagged from broken front cameras if anyone wants to help out.
(BSc Medical Electronics)
Thank you to Mike Szczys and the Hackaday team for the great article. The thing I most like about Hackaday is that we can argue about things with the community and learn something as a result.
Also see https://cromwell-intl.com/technical/samsung-galaxy/secret-code.html From memory its an 850-900nm diode 0.01W SLD so the aperture size is quite small.
Although probably not a popular opinion, Open Source medical equipment would just get snagged and copied by a large Asian country and be re-built with cheapo electronics, copypasta code, and have compliance labels slapped onto it so it could be sold as closed SoC device. Keep doing their legwork, good people :(
This has happened, i see so many of them. But how do we actually stop that from happening? One person actually suggested getting a patent for ourselves and then licensing out the patent for free. Sounds good, except for the fact that getting a patent costs more than twice our development costs and time :)
Thank you for a sensible and thoughtful answer. The patent overhead is definitely a bad deal, I really wish there was some other option. I wish I had an answer for preventing the cloning of the device outside of paying the Triad to hobble some fools haha. Good luck :)
This is actually a great idea. Innovation needs to be encouraged in the open source idea of medical device designed can only encourage that. So many people are working on devices that are actually the same technology and if people use this open platform it would get problem-solving done faster and get product to market quicker.
If only we had Open Source Respirators that could meet the COVID19 demand.
They’re literally around the corner. The UK has already released their specs of what they would use.
https://www.gov.uk/government/publications/specification-for-ventilators-to-be-used-in-uk-hospitals-during-the-coronavirus-covid-19-outbreak
Just got to build one.