It would be really hard to go through a typical day in the developed world without running across something made from ABS plastic. It’s literally all over the place, from toothbrush handles to refrigerator interiors to car dashboards to computer keyboards. Many houses are plumbed with pipes extruded from ABS, and it lives in rolls next to millions of 3D-printers, loved and hated by those who use and misuse it. And in the form of LEGO bricks, it lurks on carpets in the dark rooms of children around the world, ready to puncture the bare feet of their parents.
ABS is so ubiquitous that it makes sense to take a look at this material in terms of its chemistry and its properties. As we’ll see, ABS isn’t just a single plastic, but a mixture that takes the best properties of its components to create one of the most versatile plastics in the world.
All for One
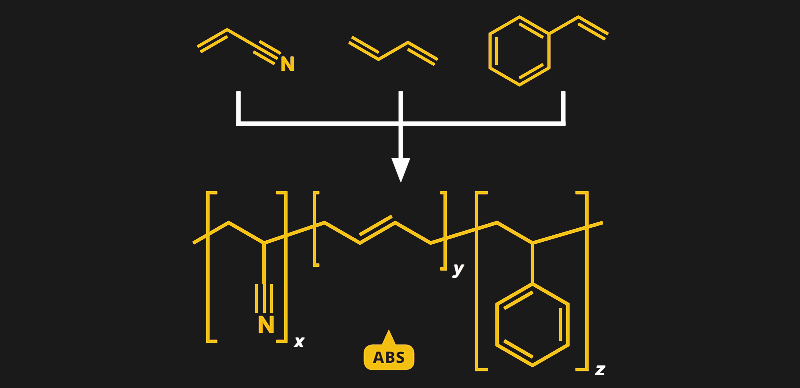
Unlike simple plastics such as polylactic acid (PLA), which we discussed earlier, ABS, or acrylonitrile butadiene styrene, is a copolymer. That means that instead of linking together a single type of monomer into long chains, multiple different monomers are linked together. In the case of ABS, it’s three monomers, and they’re all right there in the name — acrylonitrile, butadiene, and styrene.

How these three components got together is an interesting story. Back as far as the 1930s, chemists in the still-infant polymer industry were experimenting with both polyacrylonitrile (PAN) and polystyrene (PS). PAN was an early success in the search for synthetic fibers, resulting in such products as Orlon acrylic. The synthetic fibers, as well as other copolymers of PAN, found their way into everything from clothing to carpets. Polystyrene was also commercialized around the same time, initially as a replacement for zinc in the die-casting of small parts. The clear plastic was hard, but brittle and with a low melting point.
By the 1940s, polymer chemists had figured out that copolymerizing styrene and acrylonitrile monomers would result in a plastic that was still clear like polystyrene, but less brittle and with better thermal properties. The plastic was called styrene-acrylonitrile (SAN) and found use in the food packing industry, and for making a variety of consumer products.
While SAN was better than pure polystyrene for many applications, it still lacked enough toughness to be used for some applications. So in the late 1940s, chemists decided to add another polymer to the mix: polybutadiene. Polybutadiene, or synthetic rubber, had been around since 1910 and has many of the properties of natural rubber — flexibility, ductility, wear resistance — leading to its use in everything from motor vehicle tires, to seals and gaskets, to golf balls.
Add a Little Rubber

Butadiene seemed like a natural addition to the polymer soup of SAN, and in 1948, ABS was invented. With the strength of acrylic, the hardness of styrene, and the toughness of butadiene, the copolymer had a lot of new applications waiting for it. This was mainly due to the ability to control its properties by changing the mix of the three components. The plastic can be optimized for the application, and for the manufacturing method — tweak it a little and you’ve got a plastic with great properties for extrusion, allowing such products as ABS pipes and 3D-printer filament. Change the mix and the plastic works better for injection molding, resulting in myriad parts small and large, including, of course, LEGO.
It would be a mistake to think that ABS is simply three plastics melted together, though. ABS manufacture is a complicated process that has changed markedly over the years. Initially, an emulsion process was favored, where butadiene was first batch polymerized in an aqueous solution; acrylonitrile and styrene monomers were then added to the synthetic latex soup and allowed to polymerize. Little particles of ABS would form in the liquid, which could be extracted through centrifugation.
The mass polymerization method is favored now. In it, polybutadiene is produced separately, chopped into tiny fragments, and mixed with the acrylonitrile and styrene monomers. The batch is vigorously mixed during polymerization, which shears the polybutadiene chunks into even smaller sizes. The degree of mixing controls the size of the rubber particles and therefore the toughness of the plastic. It also controls the glossiness of the finished plastic, which is always very glossy when produced by the emulsion method since the liquid latex emulsion results in very small butadiene particles.
New ABS for Old
The market for ABS is huge. About 10.8 million tons of ABS resin were produced in 2016, and demand increases as new consumer products take advantage of the wide range of properties that can be dialed into the resin by changing the recipe slightly. Production of ABS is resource intensive, though. All of the monomers are derived at least in part from petroleum, and takes a lot of energy to create. Luckily, ABS is very recyclable, with used plastic being shredded and mixed to virgin ABS to form new pellets of almost the same quality of new plastic.
[Featured images credit: Alan Chia [CC BY-SA 2.0], via Wikimedia Commons]
















Interesting, thanks!
BTW: Lego is frantically looking for a more eco-friendly material to replace the ABS they use.
*or cheaper…*
while keeping the same retail prices, or higher.
I wouldn’t worry about the cost of materials with LEGO. Thier prices are so divergent from that cost that even a 100% increase in materials cost would make minimal difference to their bottom line. Remember, a wise merchant/manufacturer may look at cost to make sure they are making profit but they set the price based on what the market will carry instead. Like Coca-Cola, put 1 cent of sugar water in 2 cents of aluminum can and sell it for 69 cents (or some other typical value) because that’s the price point of greatest total profit for all items sold. :-)
While keeping the feet-destroying home-protecting sharp edges.
Learn the “Lego Shuffle” Man!!! :-) All parents end up learning it unless they already learned it as a kid. (It hurts when kids step on them too so they learn also) …never more than 2mm off the floor and you are safe …off to use the caliper on a 1/3 height piece to see if I’m right. Found a full height one… 9.6mm/3 = 3.2mm We’re good at 2mm, even 3mm if you are brave. :-)
I dont think they necessarily want to replace ABS rather they want to find a way to produce ABS (and its inputs) from more eco-friendly sources. They are already using polythene (used in flexible elements) made from sugarcane rather than oil and they are definitely looking for ways to produce ABS (for most parts), polycarbonate (used for clear parts as well as certain parts that need strength) and SBS (used for “rubber” parts like tyres) from sustainable inputs.
Even more so in the under-developed world.
Biggest problem with ABS: It turns yellow over time. When you buy the item it’s bright white, a few years later not so much.
Ask owners of vintage computers.
From my understanding that is not necessarily the ABS, but a bromide flame retardant they put in with the plastic that reacts with UV under sunlight that turns the plastic a yellow hue.
You are correct: https://hackaday.com/2017/11/03/yellowing-the-plastic-equivalent-of-a-sunburn/
Isn’t bromide the nasty stuff, banned in EU as it ends up in the body with awful side effects?
Bromide just means it contains bromine, eat some sea salt or seaweed salad and you’ve consumed bromide salts. The classes of chemical that are being banned are halogenated (usually cyclic) hydrocarbons used as flame retardants, particularly PBDEs (poly-brominated diphenyl ethers) due to their bioaccumulative nature.
Gotta love how Mountain Dew used to contain brominated vegetable oil. :P
The EU will ban water soon. I’m pretty sure about that.
I think the author means “with the strength of acrylic, the hardness of POLYstyrene, and the toughness of POLYbutadiene”. Styrene is a liquid and butadiene is a gas.
Because the butadiene is polymerized first, ABS ends up looking structurally like a graft terpolymer, rather than the random mix of monomers suggested by the chemical structure in this article.
One interesting property of ABS that results from its method of preparation is that it cannot fully be dissolved, even in good solvents. You end up with a milky emulsion-like liquid with very small particle size. There is cross-linking in the polymer that prevents full dissolution.
“on carpets in the dark rooms of children around the world” – carpet (floor)s are not around the world(nor are shoes worn inside).
where tile is prevalent… carpet rugs are as well. A home with hardwood or tile floors without any fabric is incredibly noisy.
Strangest comment ever about an article on polymer chemistry! Kudos.
But really, carpets are everywhere.
If ABS is both so modular but also “very recyclable, with used plastic being shredded and mixed to virgin ABS to form new pellets of almost the same quality of new plastic”, then how do they control the output ABS end properties when they are mixing in random ABS pellets that likely contain a mixture of ABS raw materials at various ratios?
Clearly they limit the amount added to virgin resin but if you are trying to make a defined mixture, about the worst thing you can do would be to mix in random set of noise.
That’s exactly why plastic are mostly not recycled in their original form and usage.
As an example, plastic bottles are reused to create fibers, but almost never for new bottles.
An other point is car industry.
Almost all the plastic used in a car is brand new, because they want to control the quality, aspect and ageing of the product.
Most plastics (but no resins) are highly recyclable. But most aren’t because you can’t sort them easily.
ABS and PS are the most reused, but event there, you need to put aside contaminated plastic (Bromide is a fairly common avoided component, like [sjm4306] said, but there are others).
Finally, try to match a color with recycled plastic and you understand why it’s not economic today.
I guess I just get tired of the greenwashing and ideas that, even here “used plastic being shredded and mixed to virgin ABS to form new pellets of almost the same quality of new plastic”.
Well, sort of but not exactly.
It’s generally better to recycle than to not recycle but recycling doesn’t magically create brand new monomer mixes that are the same as virgin resins or fully interchangeable either.
Plus the recycling process by nature creates waste and uses energy as well. Many average people outside the industry don’t understand things like the fact that that virgin resins only need a very small amount of pigment loading to completely color a plastic being injection molded.
How much recycled ABS is used to make LEGO blocks? https://www.wired.com/story/lego-sustainable-bricks/ It’s at least something people are working on but it is a very hard problem to easily or cheaply “solve”.
Most recycled plastics in automotive use are used to make parts where they won’t be seen or will be finish coated. They also get used for parts where strength isn’t super important or where the part can be over-built to account for variances in strength of the recycled+virgin plastic mix.
Yet another use for recycled plastic is in laminates where the core can be partly or fully recycled with virgin plastic layered on one or both sides. Most PETG beverage bottles are of quite complex construction. Preforms are molded with a thick layer of virgin PETG on the inside and outside. In between there is recycled PETG and one or more layers of plastics designed to better block oxygen from getting in (especially important for beer) and C02 from getting out.
That multilayer construction makes it difficult to recycle PETG bottles into printer filament.
The preforms have the neck and threads molded in finished form. They then get clamped into heated molds and hot air (or other gas) is blown inside the preform to form the bottle.
If we made the right products out of ABS we wouldn’t even be inclined to recycle them. How often do we recycle our LEGO bricks? I’m sure there are some who do but most keep them and reuse them forever. If we made large, super-versitile modular ABS (Nylon, or PLA) parts that were just as flexible we could build our own furniture, shelves, tables, etc and could just add or remove parts to change their dimensions. When we get tired of something we could just take it apart and store the parts until we make something else out of them. The parts could beven be bought and sold and only recycled when broken or worn out. Now that would make a lot of sense once we get used to the design constraints which may be less limited than you think. ???? (yes, these are the words of some-one who spent a HUGE amount of time playing with LEGO bricks as a child. – to me the blockiness is beautiful)
You could have been doing that with Lego bricks for the last 50 years. But people care about the way things look more than the way they function, so they don’t. It would also be extremely expensive to use Lego bricks for that purpose, but there are cheap, similar alternatives. I’m sure that there are dozens of Chinese companies that would be happy to injection mold several million bricks for you for only a few pennies per brick.
What’s needed is some very large blocks to get the bulk structure of the piece of furniture and then some interface pieces that allow much smaller pieces to fill out the details. Some fillet pieces for the corners would be nice, too.
The article is probably referencing the ABS recycling that is done internally within the company. Since a company would be recycling their own product, they can keep their plastic composition consistent and “pure”.
That isn’t quite the same as the recycling most people think of. As the article hinted at, the composition of ABS likely varies greatly depending on what it’s for and how it’s used.
“With the strength of acrylic, the hardness of styrene, and the toughness of butadiene” sounds like a description of a 1940’s comic book superhero.
“It’s… POLYMERMAN!!”
Can anyone tell me why my ABS injection molded parts come out brittle. I have dried the ABS but they still come out brittle.
TENTE also is made with ABS.