While the homebrew rebreather the [AyLo] describes on his blog looks exceptionally well engineered and is documented to a level we don’t often see, he still makes it very clear that he’s not suggesting you actually build one yourself. He’s very upfront about the fact that he has no formal training, and notes that he’s already identified several critical mistakes. That being said, he’s taken his rebreather out for a few dives and has (quite literally) lived to tell the tale, so he figured others might be interested in reading about his experiments.
For the landlubbers in the audience, a rebreather removes the CO2 from exhaled air and recirculates the remaining O2 for another pass through the lungs. Compared to open circuit systems, a rebreather can substantially increase the amount of time a diver can remain submerged for a given volume of gas. Rebreathers aren’t just for diving either, the same basic concept was used in the Apollo PLSS to increase the amount of time the astronauts could spend on the surface of the Moon.
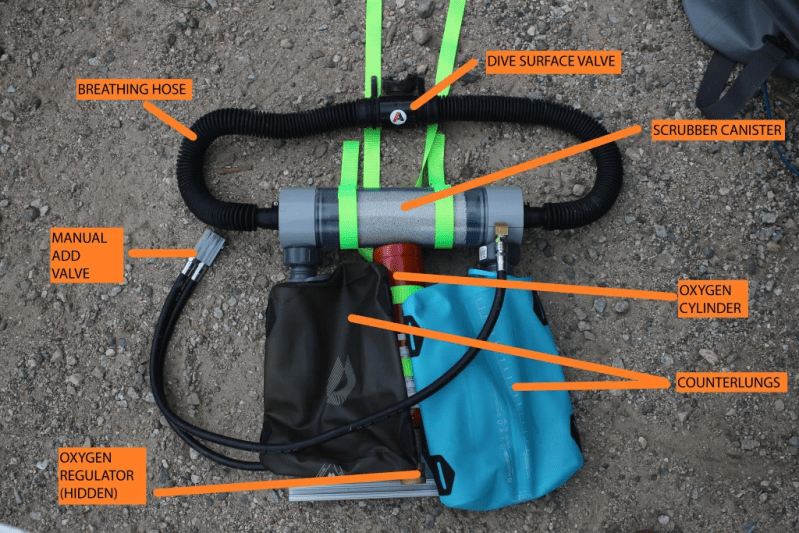
The science behind it seemed simple enough, so [AyLo] did his research and starting designing a bare-minimum rebreather system in CAD. Rather than completely hack something together with zip ties, he wanted to take the time to make sure that he could at least mate his hardware with legitimate commercial scuba components wherever possible to minimize his points of failure. It meant more time designing and machining his parts, but the higher safety factor seems well worth the effort.
[AyLo] has limited the durations of his dives to ten minutes or less out of caution, but so far reports no problems with the setup. As with our coverage of the 3D printed pressure regulator or the Arduino nitrox analyser, we acknowledge there’s a higher than usual danger factor in these projects. But with a scientific approach and more conventional gear reserved for backups, these projects prove that hardware hacking is possible in even the most inhospitable conditions.
















neat Ive never looked at a scuba rebreather before but looks just like the way an anesthetic machine operates, just without the ventilator to drive the breathing.
And – more important – without the anesthetic drugs. :-) You don’t want to get unconscious under water. Nitrogen narcosis is a threat in greater depth.
But I see only an oxygen supply, no dilution gas like nitrogen or helium. You must not use pure oxygen in greater depth than about 15 to 18m. At too high partial pressure of O2 lung and CNS damage is possible.
Pure oxygen has a maximum operation depht at 6m (at a partial pressure of 1.6 bar). But he is using an sensor for monitoring the o2 ratio in the circuit and dont want to breath it pure. So the main problem will be the narcosis and the solved nitrogen in blood at greater depht.
15m is quite brave. Ambient pressure at 15m is 2.5bar, which gives you a PPO2 (partial pressure of O2 of 2.5).
Most people think 1.6bar is a more reasonable safety limit, which is achieved at 6m…The biggest risk, or at least the one that will kill you quickest is a hyperoxic seizure, causing your death by drowning.
Hey all! A clarification- no O2 sensors in this thing, the inhaled gas is ~100% O2. I limit the dive depths to 20ft/~6m to avoid O2 seizures/O2 toxicity.
I think your greatest danger is letting CO2 level go wrong. This controls your breathing reflex and has meant the end of many a diver.
My concerns are that your scrubber canister is too small. As those are used they get wet and air channels start to form cutting back on the absorption of CO2. Many brands of scrubbers (baralyme and Sodasorb) have indicators that turn purple as the scrubber is used up. I don’t see a way you are able to monitor that from its placement behind your head. Also be aware that when that media dries out the color goes away(pH indicator) so don’t rely on looks to tell you if the media is used up.
An oxygen sensor is not optional in a rebreather. Stop and correct that please.
Ok, think I have to read the real site next time and not only the post on hackaday :-)
I continue diving with bail out system and live with the limited gas supply of my tank I can take with me.
Good discussion! I only dive this to 6m/20ft to limit the PPO2 to 1.6, as there is no O2 sensing. This depth limit is to prevent O2 seizures (which happen at high PPO2s, especially past 2.0, according to literature). Short dive times limit exposure to oxygen, and therefore help to prevent chronic O2 toxicity. Oxygen seizures seem difficult to survive underwater, especially alone and without a FFM.
Interestingly, there is a long history of O2 rebreathers being operated at greater than 6m. The person that I think of as the quintessential rebreather diver is Hans Haas, who used it in the 50s for underwater photography and exploration. The rebreather he used, the modell 138, was “specified” to operate at around 20m/60′- a PPO2 of 3 (yikes). Drager (much) later decided to restrict diving to the now-standard depth limit of 6m, which is the limit used by most recreational O2 rebreather manufacturers today (like the italian OMG).
O2 toxicity susceptibility is poorly understood and seems to have a lot of environmental and physical factors, but limiting depth to 20′ seems very conservative and leaves a wide margin of error (in terms of feet) from the theoretical limit of PPO2 2.0. Unsurprisingly, people found out very quickly about O2 toxicity in the early development of rebreathers, and there are rumors (unsubstantiated in my research) that early divers like Haas actually left a little normoxic air in their rebreathers before turning on the O2 to pad their PPO2.
You can actually watch a lof of the old rebreather dive films on youtube. To put it gently, some of it has not aged well but the photography and technology are pretty amazing. Here is Hans Haas in the red sea: https://youtu.be/HbipiF3t2RQ
Hans Hass used a technique to dive reasonably safely to 20m by starting with the counterlung and his own lungs full of air, that reduced the PPO2.
Indeed he did! I did the same thing with a later revision of the rebreather, as you can read about here https://aylo6061.com/2020/09/27/little-pond-big-dive/. I also added a PO2 display and sensing.
I’m diver since early 90’s.
I have some experience with rebreathers. If I learnt something is that this things are not toys.
Many people with training and experts in diving with these devices have died.
Hypoxia and hyperoxia are the most frequent problems you can find while diving with a rebreather.
A system that measures the oxygen of the mixture that you are going to breathe is vital.
I really like that people investigate and learn but please with these devices the possibility of a very serious accident is very high
Anyway congratulations to the builder. A functional rebreather, included in its most basic form is not easy to build
Agree.
I’m not sure I would ever trust my work to that level, especially if it’s a novel design. I would feel way more comfortable building and flying a kit aircraft — at least with that, there are a lot of things that can fail and still leave you able to land safely.
With this, everything is mission-critical.
Having said that, I have massive respect for this endeavor; mankind wouldn’t advance if everyone always took the safe, well-beaten path. I just worry that he’s rolling the dice every time he uses this, especially if he’s solo diving.
Do I see a little brazing torch O2 bottle, I do. Which aren’t filtered, and have pretty dirty gas.
I’ve tried to use these bottle for experiments before instead of hauling out one of my medical O2 bottles
And they have had to many contaminates. Its scary that he is using one for breathing let along breathing underwater.
you certainly do see a little brazing torch O2 bottle. Unfortunately, getting a reasonably-sized medical O2 cylinder (that will be possible to refill) has been a challenge. Out of curiosity, what contaminants did you find in the brazing bottles?
One of the differences between clean and ‘not clean’ gas is the way they store the bottles between fills, impure gas tend to get stored open and as a result may have what is technically known as “a massive pile of rusty crap” in the bottom of the bottle.
Supposed to use dry oxygen for that very reason. At pressure water and O2 are very aggressive.
There is no unclean O2… you get the industrial and medical oxygen from the very same supply. If a tank is used for dirty air, for example filled with normal compressor without filtering, it can never be used for pure oxygen anymore. In theory, it can be cleaned again, but a decent dive center would not take the risk. If any oil residue stays in the somewhat porous material of the tank, it can do nasty things. You MUST store your O2 clean tanks properly.
While I wouldn’t encourage long term breathing of technical grade oxygen, whether it’s O2 or compressed air, it all comes from the same medical grade bulk tank. The only difference (in the US) is the tank filling protocol.
Compressed O2 especially is really pure. There’s a reason O2 fittings say ‘Use No Grease’. At 100% O2 partial pressure most things combust, so whatever ‘dirt’ may be in technical grade oxygen it’s very well oxidized and probably not an immediate concern.
>most things combust
I’d fix that a little – even at 100kPa partial O2 pressure almost everything even remotely flammable in air combusts VERY vigorously, some downright explode. Pushing the partial pressure even further results in many flammable things becoming explosive…which is why I think that technical grade oxygen can’t be all that dirty, as it would turn the pressurised cylinder into a bomb.
So all the oxidised crap (smoke, soot) won’t be a problem? So if there would be sulfur contamination inhaling sulfur dioxide would be OK? I don’t think so.
These are factory new cylinders not mystery cylinders from a scrap yard. I’m not saying they’re medical grade but where is this soot / carbon/ sulfur coming from? All of which would cause safety concerns at the factory if they didn’t clean them out prior to filling.
Again, I wouldn’t suggest regularly using technical grade O2 but once or twice isn’t going to cause you issues and certainly isn’t the most likely thing to kill you in this build.
Comments on this will be interesting. I personally DGAF if you wanna kill yourself with a razor or with a poorly executed project, just make sure you know what you are doing to those who care about you. Also personally, I can get behind the insane geniuses who experiment with and on themselves for science, but this seems silly. We already know how rebreathers work and we already know how to build (relatively) safe ones. Why take the risk? At best you save a couple bucks, at worst, well, you know.
I am a diver for a long time and I have dove a rebreather before. A few points:
1) You will die! If you don’t have a CO2 sensor and an O2 sensor you will die! The problem without the CO2 sensor is that you will not even notice when you will die! You will go to sleep from too much CO2 and then wel … you will die.
2) The rebreather’s point is to enrich the closed circuit of the re-breathing with O2 when needed. So you take a breath in, you blow the air out, the scrubber (the salt that he has in the canister) scrubs the CO2 out and the oxygen tank adds a little bit more Oxygen in the mix because you used some in your last breath. You do not breath in pure oxygen so there is not a problem with the Oxygen toxicity. Rebreathers are designed to have (almost) no depth limits.
3) There is no nitrogen narcosis here since … well, you don’t add any more nitrogen in the mix. What you took in when you put your head under water is what you will have, that’s also why you will not have huge decompression times when you come up after a deep and long dive.
Seriously guys, if you want to try this in a pool, do it. Pack a scrubber in a bottle, add a counterlung on the top of it and add a cannula with very little oxygen leaking in through the scrubber … but don’t do this in open water or at depth!!!! The professionals die on rebreathers. You don’t pack your fresh scrubber – you die. Your sensors fail – you die. You don’t have a bail out bottle – you die. You bail and you don’t have enough bail out gass – you die. You have a leak in the system, the scrubber salt reacts with the water – you die.
Stick with SCUBA it is awesome. Yes, the bubbles will scare the fish a bit but totally not worth it yet.
Vlad – while I’m sure you’ve dove a rebreather before, please be aware of some critical issues with your comment:
1) There are no reliable commercially available CO2 sensors for the ppCO2 that is hazardous in the breathing loop. There have been attempts at making them but non so far have been reliable by any stretch. Regarding the O2 sensor, this is an O2 only rebreather, and not a mixed gas unit like most common scuba ones these days, which involve both a pure O2 cylinder and a diluent (air or norm/hypoxic trimix). There are also semi-closed rebreathers which operate on air/nitrox/trimix which only use a diluent and basically work as gas extenders (removing CO2 and adding new breathing gas once the ppO2 drops below a set level).
2) For a mixed gas CCR, you are correct. However as stated this is a pure oxygen rebreather so the only diluent will come from the diver’s lung contents, and it’s safe to assume those are quickly voided and outweighed by the continuous addition of O2 via the needle valve. So the max ppO2 of 1.6 atm is an appropriate safety guideline. The NOAA diving manual has published tables for O2 exposure vs time which allow for some bending, but you’re more likely to get an O2 hit under heavy breathing or stress.
3) This statement is generally correct – no nitrogen means no narcosis. Though there is still a debate in certain circles as to if oxygen can be narcotic – the conventional wisdom is that metabolic gas is not but there’s a reason the debate lives on. Narcosis is very difficult to quantify and has different effects between people.
Every rebreather is a possible death machine but so is any other form of SCUBA – just rebreathers tend to fail silently and you just doze off rather peacefully.
There’s a great history of rebreathers here: http://www.therebreathersite.nl/
Also, for O2 rebreathers, check out ‘yoyo’ or pendulum type rebreathers, they’re a bit simpler on the part count and pretty close to what was built here.
Andrew Goring / Sump UK has been developing his own for a bit and documenting it somewhat on Facebook – it’s worth checking out to see other takes on the same idea.
Good luck and keep diving!
You have a few contradictions here.
He does ultimately end up breathing pretty close to pure O2. The scrubber removes CO2, and he’s only adding back pure O2, so aside from the NO2 in the loop/his lungs/blood when he goes under, he’s not breathing any more.
He most certainly does have a potential problem with O2 toxicity, which is while he is smartly limiting his depth.
And rebreathers that don’t have a depth limit (within reason) contain an O2 bottle and a diluent, which are continually mixed and monitored to keep PPO2 within safe limits for a given depth. This setup has no diluent and no monitoring, so he, again, ends up breathing pretty close to pure O2… so his only means to limit his PPO2 is to limit his depth.
I applaud his ingenuity and courage, but do question the risk/reward ratio. Professional, commercial rebreathers have and continue to claim lives, rolling your own is seriously risky. I would hope/suggest he only uses it in the company of another diver on open circuit gear who could render assistance if something goes wrong.
Yes, this one is pretty high on the bad case of death scale. Mixed gas rebreathers have an inert gas source to keep the PPO safe, and CO2 monitoring, etc. Beckman bought rights to the Electrolung back in the 1970. No computers and pretty safe IIRC. Used routinely at 400 feet I think it was eventually rated at 600. It had triple O2 sensor and CO2 sensor and a mostly analog controller(?). http://www.therebreathersite.nl/11_Closed%20Circuit%20Rebreathers/electrolung.htm Modern ones are pretty impressive, but bigger. Still, you can find video of professional divers dying on a rebreather for no apparent reason but inattention -suddenly yelling into masks and spinning in circles and convulsing. Not pretty.
needs to be automatic, transparent. You can build analog controls, pretty much all were before NASA funded development of integrated circuits. Digital now because it can have preprogrammed responses to varying conditions, more complexity
He inhales normal air at 78% nitrogen and 21% oxygen,
exhales 78% Nitrogen, 16% oxygen, and ~4% CO2
The scrubber strips the CO2
and the difference in volume is spiked into the residual exhaled gases….
Theoretically in a perfect world with meters and measures replenishing it to roughly the original composition
Pure oxygen IS ADDED, But at no Point is he breathing PURE Oxygen.
Problem is you’d have to precisely meter the gasses – this is hard when the ambient pressure keeps changing as you swim up or down.
You’d either need several very precise pressure sensors and a computer (many fail points) or make the entire thing mechanical (still many fail points + a nightmare to design, build and maintain)
…….. its not that hard…………….. simply pressurize the area where all gasses and controls are kept……..it stays 1 atmosphere & the out put to the diving operator is regulated to depth specifications.
to continue……. the pressurized enclosure is has the o2 tank & the diluent inside the pressure case ……..everything is inside the pressure enclosure…… with the life line of breathing gas regulated to the driver operator………..
the dive pack ( if one can call it that ) is totally pressurized.
with the mix regulated for each depth attained .
Given the myriad things that can go wrong, from convulsing to shallow water black-out, why not just nitrox? You get long dives with modern high pressure tanks, plus feel 10 years younger. On the other hand, I really wanted one of those Evinrude floating hookahs when I was a kid and rebreathers were fascinating.
I’m a rebreather diver – CO2 sensors are very rare, and usually arent in most recreational rebreathers.
Pure oxygen rebreathers dont usually have or need O2 sensors, as provided you purge it thoroughly, that’ll be the only loop gas (assuming you checked your O2 bottle contains pure O2)
I *am* concerned about scrubber size – at higher minute volumes, the gas dwell time might be short enough that breakthrough of unscrubbed CO2 could occur, which is very bad indeed..
If you’re looking for more amateur diving gear, built when Poland was governed by communists and shelves were empty then check out small museum of diving in Warsaw https://en.wikipedia.org/wiki/Museum_of_Diving,_Warsaw
amazing stuff given realms of live in soviet bloc.
Mine Safety Equipment has rebreathers for mine rescue. The O2 is stored as a chemical and is quite compact.
When is O2 *not* a chemical?
I think he means they use perchlorate based oxygen candles instead of gaseous O2 in their storage systems.
Everyone’s talking about issues with the scrubber failing and the diver not knowing. Can someone explain this? Excess CO2 usually increases the breathing response, so presumably you’d know pretty quickly as you’d be gasping down air breathlessly.
The symptoms you describe of quietly going off to sleep sound like CO poisoning.
I’m guessing the pressure affects something?
While that’s the intuitive response, due to the higher partial pressures being breathed at depth, the typical failure mode as observed is the ‘fail sleep’ where you very peacefully nod off. Sometimes you feel a little funny if the ppCO2 creeps up but you don’t always notice it, especially if stressed (like fighting a heavy current). Many newer mixed gas CCRs have a BOV ‘bail-out valve’ on the mouthpiece that switches you back to open circuit SCUBA – it’s a good way to check with some sanity breaths if you think something funny is going on.
While scrubber being fully used is a failure mode, another common one is channeling – where the scrubber settles in such a way when not fully packed that the recycled gas is able to make the round trip without having the CO2 removed. Lots of focus on packing when you’re refilling your scubber.
https://en.wikipedia.org/wiki/Hypocapnia / Hypocarbia is well researched and understood. How do O2 rebreathers protect against it? It seems like one of the myriad “bad ways to die” available to divers…
Don’t let Kent see you testing it or he’ll tell Dr. Hathaway.
When your hacqualung makes you hack a lung.