In the race toward a future free from fossil fuels, hydrogen is rapidly gaining ground. On paper, hydrogen sounds fantastic — it’s clean-burning with zero emissions, the refuel time is much faster than electric, and hydrogen-fueled vehicles can go longer distances between refuels than their outlet-dependent brethren.
The reality is that hydrogen vehicles usually need fuel cells to convert hydrogen and oxygen into electricity. They also need pressurized tanks to store the gases and pumps for refueling, all of which adds weight, takes up space, and increases the explosive potential of the system.
Kurt Koehler has a better idea: make the hydrogen on demand, in the vehicle, using a solid catalyst and a simple chemical reaction. Koehler is the founder of Indiana-based startup AlGalCo — Aluminium Gallium Company. After fourteen years of R&D and five iterations of his system, the idea is really starting to float. Beginning this summer, these pucks are going to power a few trucks in a town just outside of Indianapolis.
Pucks for Trucks

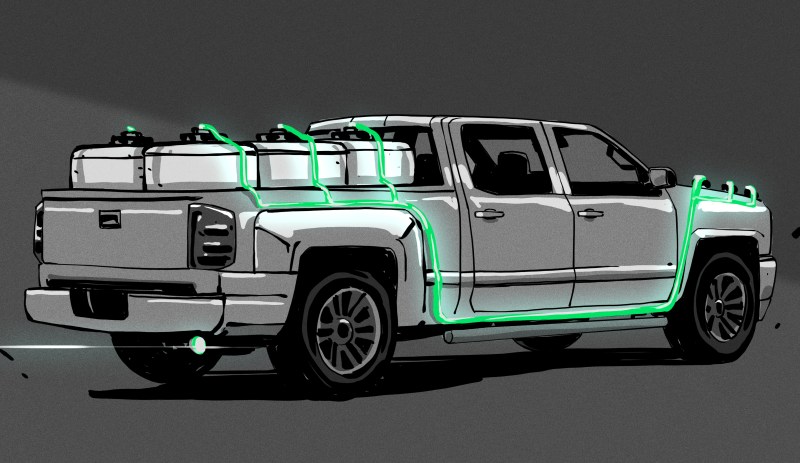
AlGalCo’s hydrogen-on-tap (HOT) system has none of the bulky and dangerous trappings of other hydrogen vehicles. Instead, it uses solid pucks of an alloy of aluminium and gallium to instantly create hydrogen whenever it’s needed. The moment these pucks come into contact with water, a chemical reaction begins, and the water molecules are split into hydrogen and oxygen. The resulting hydrogen gas is captured and sent immediately to the intake manifold to fuel the engine. Here’s the best part: the oxygen binds with aluminium and becomes aluminium oxide powder, which can be turned into new pucks indefinitely with the addition of more gallium.
This summer, the city of Carmel, Indiana is running a trial on five of their street department’s existing gasoline-engine trucks. These trucks will be retrofitted with the hydrogen-on-tap system, which consists of a metal box with six canisters that sit in the truck bed just behind the cab. The hydrogen is pumped underneath the bed and into the engine. Each day, the trucks will start out running on hydrogen and automatically switch over to gas when all the canisters full of alloy pucks are spent. When they roll back into the motor pool, the canisters can be swapped out for fresh ones in a matter of minutes. Testing has shown that the system brings a 15% improvement in gas mileage to these trucks, and a 20% reduction in carbon dioxide emissions.
A Solid Solution

Kurt Koehler has been developing this hydrogen-on-tap system at Purdue University for the last 14 years, but the technology itself is much older. The HOT system is based on the work of Jerry Woodall, who discovered the chemical reaction in 1968 while he was developing aluminium gallium arsenide compound that gave us the cheap and energy-efficient red LEDs now used in brake lights, traffic lights, and DVD players.
Woodall was trying to grow crystals in a solution of aluminium and liquid gallium. When he rinsed out his crucible with water, there was a violent, gas-emitting chemical reaction. That gas turned out to be hydrogen. Over time, he tried using less gallium.
Below is part one of a two-part video of Kurt demonstrating the previous iteration of the HOT system, including the awesome chemical reaction that goes on inside an AlGalCo canister. (Link to part 2)
We think this is a fantastic step toward widespread adoption of hydrogen power, and the hydrogen-gasoline hybrid trials will probably win the idea a lot of support. Solid fuel is easier to store and transport, and this particular fuel seems to be non-volatile as long as it stays dry.
All five of the Carmel city trucks should be running by the end of June. If you happen to live anywhere near central Indiana, Koehler gives weekly demonstrations of the technology at the Carmel street department’s garage. AlGalCo plans to adapt the system for use in diesel trucks and delivery vans, and already has interest from New York City officials.













Is Gallium that cheap? This becomes a gallium fueled system right?
According to https://phys.org/news/2007-05-hydrogen-aluminum-alloy-fuel-cells.html (13 years ago?)
“No toxic fumes are produced,” Woodall said. “It’s important to note that the gallium doesn’t react, so it doesn’t get used up and can be recycled over and over again. The reason this is so important is because gallium is currently a lot more expensive than aluminum. Hopefully, if this process is widely adopted, the gallium industry will respond by producing large quantities of the low-grade gallium required for our process. Currently, nearly all gallium is of high purity and used almost exclusively by the semiconductor industry.”
I am not sure that Hydrogen produces zero pollutants when burned in a combustion engine. I would anticipate NOx being formed by the same processes as are seen in conventional engines. (the NOx is formed from atmospheric nitrogen and oxygen)
Piping the gas from this process to a fuel cell seems like it might be adding an unnecessary stage. This is a redox reaction, and it feels like it ought to be possible to use this directly as a fuel cell.
Yep. Many people have been trying to develop the direct aluminium battery, which is what you’re talking of as a “fuel cell”, and it kinda sorta works but the failure point is always recycling the aluminium back – it costs too much since there’s no nice and clean reactions to reduce the oxide back to metal.
You basically send the resulting alumina back to the smelter, where it is heated red hot and electrolyzed into a billet on top of a carbon electrode, and the whole process of shipping what’s basically aluminium sand and metal ingots around is inefficient.
It wasn’t quite clear until the end of the video how they are using the hydrogen. I expected it would go to a fuel cell for an all electric vehicle. But that would require a LOT of hydrogen – hydrogen has a MUCH lower heat content per volume than gasoline! All they are doing is injecting the hydrogen into the intake manifold to supplement the usual gasoline fuel. I would think that the percentage of hydrogen/gasoline mix is fairly low, so I don’t think this would make much of an environmental impact and is likely expensive and inefficient. Maybe the cost can be justified as advertising cost to be”green”.
They hose it in the intake, so the car idles partially on hydrogen and the ECU cuts the fuel supply to compensate.
It is a gimmick.
Aluminium–air batteries nothing new and can be commercially purchased, it isn’t widely used because it is just an expensive primary cell, however Al-air batteries can be used as fuel cell with design which allow replaceable anodes, otherwise it still just an expensive primary cell.
https://en.wikipedia.org/wiki/Aluminium–air_battery
There’s similar rechargeable option:
https://en.wikipedia.org/wiki/Aluminium-ion_battery
Also there’s much more interesting option of replacing fuel cell by secondary cell with similar capacity:
https://en.wikipedia.org/wiki/Lithium–air_battery
This is not even remotely aluminum air. That actually makes sense. This is using an aluminum alloy to make hydrogen on demand that is then fed into a combustion engine. Even using the hydrogen in a fuel cell would have a well to wheels efficiency of about 20% taking the aluminum refinement into consideration. Using it in combustion is more like 7% well to wheel efficiency. This is a dumb gimmick. Also aluminums melting point is a tad over 1200F. Using a glow plug on a solid pure aluminum ingot for a second and allowing a molten drop to interact with water would allow a heat engine fuel cell hybrid with more efficiency and is far more simple. Either way using aluminum to produce hydrogen is stupid. Hydrogen production by waters reaction with a metal is all about atom for atom. Sodium metal is less then half the density of aluminum and packs almost 8 times less energy. Which means you get the hydrogen using 8 times less energy. Aluminum air makes sense. This is ridiculous.
I get that they’re trying to reinvent calcium carbide but isn’t it already cheap enough?
https://www.climatecolab.org/contests/2016/transportation/c/proposal/1331105
So this method of Dr. Jerry M Woodall is more efficient then gasoline. It is around 25% efficient used in a combustion engine and around 70% efficient used in a fuel cell. Everything is recoverable except the Hydrogen that comes out of the Aluminum. To remake the Aluminum one can use the newer electrodes that last much longer then the carbon. If one was to use the Aluminum to store energy like in a battery one would not have to worried about the energy getting lost as modern day batteries have a self life. So the energy from a solar array could be used to covert the purified Alumi powder back into Aluminum and the energy will stay there forever. You should look up the inventor of this discovery at this place and look up his patents and history. https://woodall.ece.ucdavis.edu/people/jerry-woodall/
No, it is powered by the electricity that is used to turn the aluminum oxide back onto aluminum. Unfortunately that is about ten times as much electricity as a battery powered vehicle would require. Solar and other green energy sources are not free and their output should not be squandered on low efficiency processes such as this. This may have applications for a small market of long range limited support infrastructure vehicles (military), but only batteries are efficient enough for the majority of the automobile and truck market.
You should contact Dr. Woodall. As I remember, he claims that it would cost 2 cents a kilowatts hour to turn the aluminum oxide back into aluminum. The cost of creating coal to aluminum perhaps is expensive but, once it is aluminum then switching back and forth is not a great expense. Also, it would be good to look up the cost of the elements in the rechargeable batteries and their cost. Lithium I do not believe can be recycled as it is lost. I have no idea how much it cost to make lithium for batteries. Consider the weight of the rechargeable batteries in the vehicle which lowers the efficiency and then what does one do the the waste after they are used?
What happened to the HHO generators? Or why not make a hybrid kit were you install an electric motor to the pulleys like the alternator and 8nstall a controller box and a lithium battery? Something like the ECharger but more wallet friendly.
Does it really qualify as a catalyst if it is spent during the reaction?
nope
The gallium appears to be the catalyst, while the aluminum is the fuel, and hydrogen is the waste product.
It would be more accurate to say that the water is the fuel. The aluminium is a reactant and since the goal is to produce hydrogen, hydrogen is the product, not “the waste product”.
No, the water can not be considered as fuel. This would be the same as if you call the intake air of a gas engine “fuel”. It is the aluminium.
Actually the fuel is partially carbon – the electrodes for the aluminium electrolysis are consumeables – and partially the electricity needed for this process. Then the aluminium and the hydrogen are intermediate energy carriers. Over all an ineffcient “rube goldberg machine like” process.
Smelters that use new inert anodes made from copper alloys will not produce CO2.
What is wrong with electro-fuels? Normal fuels also have horrible efficiency because of the refining.
Electro-fuels have a lot more energy density compared to batteries.
Batteries works when the traveling distances are short, hauling is low and you have access to a charger at home/base. You will not see electrical vehicles on Gold Rush but you might see electro-fueled ones in the future is my opinion.
Normal fuels actually have relatively small efficiency losses due to refining, and it’s a moot point because the energy is coming from the petroleum itself. Oil is cheap.
With aluminium, you’re starting with expensive electricity and throwing away most of it, which means multiplying the cost.
A lot of “environmentalism” seems to be “push the problem outside of consumers vision”.
Save fuel by riding your bike, just ignore the fact that your food was shipped by tanker from Guatemala 3× less efficient than your car. Reduce emissions with solar power, just ignore the costs of mining and building panels.
These arent solutions they’re distractions. Anything on a slow news day I guess.
You can ride your bike AND buy local food. But even if your food is imported you have still saved your car journey – a net improvement.
Solar panels do have an environmental cost, but it’s a one-off, then the panels produce electricity for decades. Compare this to fossile fuels where you need to continuously burn them to continue to produce electricity.
The actions you specify are not the WHOLE solution – there isn’t a “magic bullet” for this problem – but they are PART of the solution, and not a mere distraction.
There certainly are examples of distractions, but these aren’t them.
The article does not mention where is the gallium going in the process? Also I though that stripping the oxide from aluminum was the most energy intensive part of the process of making new aluminum; and the reason why recycling aluminum is prevalent. And as such, how much energy goes into making these pucks, and how much comes out of the reaction?
my guess is the gallium is use as a kind of wetting agent to prevent passivation of the aluminium puck. and yeah i had the same thought maybe it dosn’t produce co2 or greenhouse gases but sure sounds inefficient as f****.
Making aluminium from aluminium oxide might not be so different from making hydrogen from water. Both use large amounts of electricity. But there probably is some loss along the way, as I would expect that the aluminium pellets get quite warm. I have forgotten how to do chemistry, but I guess the (relative) efficiency can be readily calculated from the enthalpies of the reactions involved.
This is somewhat like the electric car but yeah where does the electricity come from problem. We are good at making aluminum from aluminum oxide. You can do it very economically (and if you do it in iceland where they use abundant hydro power you can do it quite cleanly.) You can also do a terrible job if you run your aluminum refinery from a coal plant.
As Dr. Woodall says, “they are not making anymore dinosaurs to make oil.” I wonder how efficient coal or oil is? First kill, a dinosaur, millions of millions of bacteria and plant life. Then bury it 10,000 feet deep and wait for pressure and time to convert it to crude oil. Then one has to find it, drill for it, transport it and refine it. Then one has to ship it to gas stations. Once used it is gone forever.
Gallium is used to continue the aluminum oxidizing. When the aluminum oxidizes by itself, because of air, a oxidized coating is developed on the surface by given up hydrogen then the oxidation is stopped. When Gallium is added it prevents the aluminum oxidized coating from stopping. It is a catalyst and completely recoverable.
Apparently, all details and figures on whether this is viable and sustainable are also part of the secret patented sauce. We already have electric cars, thank you for trying.
But involving hard to dismantle batteries, made of multiple nano compounds. I won’t talk about the rareearth involved in electric drive fabrication. None of these solutions are perfect.
But this one seems to have less number of components which also seems less complex, involved in the energy stockage process.
Motors and magnets are easy to recycle/reuse so that’s a non-issue. We’re not far from solid state batteries, so batteries won’t be an issue for long. The mechanical complexity of an EV is much lower making it easier to maintain. It’s not perfect but it’s still the best solution thus far. The real issue is energy density because airplanes are big polluters.
Don’t call it before it’s done. Solid state batteries have real troubles working in the cold. At the present, they need to be heated up to 50-60 C just to have low enough ESR to function.
Or as Yogi Berra said,
“It ain’t over, ’til its over!”
20 years ago they were operating up in the hundreds of degrees, so by extrapolation we should have a room-temperature solid state battery some time in the 2040’s…
“At the present, they need to be heated up to 50-60 C just to have low enough ESR to function.”
lolwhat? No, they don’t. Most EVs heat the battery up to 10-20C in the winter time, and you can manage how/when/why in many of them.
>No, they don’t.
Solid state batteries refer to cells without liquid or gel (wet) electrolyte. No EV has these, because they don’t work under practical conditions yet. They’re also so expensive that you’d pay millions to have one.
There were practical molten salt batteries in use in EVs, e.g. https://en.wikipedia.org/wiki/Think_City
So heating up a battery to a couple of hundred degrees celcius is a problem we’ve solved in the past. What it meant is you couldn’t leave the car sitting for weeks at a time otherwise it’d self-discharge and need a while on the charger to revitalise.
If it is patented, it isn’t secret.
Not necessarily. A lot of people patent certain aspects then keep other parts a secret sauce. Once there is a patent, it becomes public information. But if you keep other parts secret (Art/Know How) then you can really keep your competitors at bay. You are both protected on the patents and have hidden information that they will need to research and figure out.
Yes, “it’s clean-burning with zero emissions” but the creation of the fuel is incredibly inefficient. And guess what powers most of its creation? Dead dinos.
Consumers need to be better with understanding the whole process if they really want to be “environmentally conscious”.
The same could be said for Electric cars. It is not the creation of the energy that is the problem as that is already being worked on in the form of solar and wind energy. (of course that is limited too but still something being worked on).
This system takes place of the battery component which can be thought of the same thing that gas is. Its a battery technology for storing energy. Currently the battery technology of electric cars is what is the limiting factor and what Hydrogen is trying to solve.
No it couldn’t be said about electric cars because electric car batteries last over 1000 cycles while this thing is used once before it needs to be remade. Why aluminum-to-hydrogen is particularly bad: Aluminum is smelted using electricity, but it also almost always requires consumable graphite electrodes, made using fossil hydrocarbons. The round-trip efficiency of this process (electricity to electricity) is terrible (I’d be surprised if it’s better than 20%) whereas for batteries it can be over 90%.
^^^ This guy gets it. I did a large research project on hydrogen in high school and went into it super jazzed to explain to my class how it is the next great thing. Every layer of the onion I peeled back just got worse and worse. If nothing else, it was a good lesson on how our initial views can be incorrect and we should be open to changing our minds. Hydrogen tech hasn’t really changed since then.
Indeed, though when Hydrogen was first gaining some attention Nuclear power wasn’t creating such a negative stir – so cheap, and green Hydrogen was a possibility. With the huge and mostly unjustified amount of negative press around nuclear power anything that relies on electricity is far less likely to be ecologically sound. And its no longer feasible to be wasteful converting energy types multiple times for the ease of use/storage/transport for each step.
Nothing against the ‘renewables’ of electric generation but none of them are backbone reliable. About the only energy source in that family that could be considered reliable nobody is willing to make new facilities for as it has an upfront change of land use, Oh no this heathland will turn to lake or this coasline becomes tidal marshland! – Hydropower be it dam across a suitable river or tidal systems can be controlled to a great extent and provide either perfectly predictable energy output cycles (tidal) or have a big energy store – the dam and can be managed effectively – its the battery and the generator in one.
>Hydropower
If all the fjords in Norway were dammed up, they would have an energy capacity of around 85 TWh according to some German research paper I remember reading. That would be worth about 7 days of grid demand in Germany.
Where do we find a second Norway for Denmark, France, England…
Over 1000 cycles… The electric buses we have (Both dutch VDL Citea and Chinese BYD K7u) charge on average 3 times per day and the battery pack is supposed to last 6 years, coming at ~5500 charges with an end-of-life capacity of 85 or 82%.
We also have articulated electric buses that fast-charge (240kW, 21 minutes charge time) about 8 times per day, with actively cooled batteries. I don’t know whether they are calculated to go trough 2, 3 or 4 battery packs in their 15 year calculated lifecycle though.
Lithium titanate batteries can do that – but they’re heavier and more expensive, so less suitable for smaller cars.
That’s a lot of handwaving regarding the source of electricity. We are still a long way from any sort of clean energy majority. Most of it’s nasty, dirty coal and the most growth is in natural gas which still results in a lot of CO2, at least the portion which isn’t just being vented straight into the atmosphere at a sloppy well.
people who want electric cars should also want nuclear power. because wind turbines and solar panels aren’t going to charge everyone’s cars on their own.
It wouldn’t be impossible to get power with continent spanning grids of solar and wind and pumped hydro/grid batteries/CAES to smooth the grids out, combined with some degree of energy efficient design for the consumer electronics. Everyones PC and every office PC could make a huge saving just by moving to gold or platnium rated PSU’s for example. I know no ex-company PC i’ve ever had to work on had a PSU with great efficiency, and many of the pre-builds I’ve seen for gamer are not good efficiency wise either. Making the average computer PSU probably as much as 30% more efficient would be huge.
Can’t see it happening though – too much international co-operation and i don’t think the electric storage techs are really quite there yet. Pumped hydro is efficient enough but need suitable sites. CAES is cheap, can be sited anywhere and durable but getting high efficency while possible is challenging and space consuming. Batteries have limited life and cost alot..
The difference between a really bad PSU and a platinum is rarely over 15%, and a 200w bad PSU and a 1000W platinum might even be worse when delivering 50w to an idle system, and today almost everyone in the industry use thin clients that use maybe 10 watts when going full beans watching a video or something. The big power regarding computers is consumed by monitors (Which are quickly getting a lot better. My new monitor have quadruple the amount of pixels, emits more light and still uses almost half the power of the 10 year old monitor it replaced) and servers/network-equipment, which very much have top-notch powersupplies because they are usually limited by cooling and run at high loads for years and years and also need super-clean power, which all requires a well-designed efficient PSU’s.
Creators of course need more oomf and might buy prebuilt, but these will also have efficient well-designed PSU’s for a lot of the same reasons that servers do.
Laptops don’t consume much power anyway (An old one might consume 25w average, newer ones less than 10), and the ones that do, need efficient PSU’s because the powerbricks are passively cooled, and the internal PSU most be efficient to prolong batterylife and reduce cooling-requirements so the laptop can be lighter/smaller/silent.
There is a lot of older prebuilt desktops still around that definitely could consume less power, but the older processors are really the culprits here as a 6-year old average CPU that consumes 60w then can be replaced by a CPU today that needs less than 10w (And idles a LOT lower than the older CPU. Might be 20w vs 2w), and then we have the mechanical drives, PSUs, the old monitor they probably are connected to, etc, but that’s the same discussion as old cars, fridges, heaters and so on, and unless they are using the desktop every day, i wouldn’t replace it anyway. My parents have on i’m sure i spend more time fixing software-problems on than they are using. They basically only use it when they have to print something, and everything else is done on their tablets or phone.
Agreed Mathias there are lots of other small tweaks that make a difference – But I’ve seen pretty much new prebuilds using horribly inefficient PSU’s so its an easy fix to prevent wastage that leaps out at me.
New processors in old machines probably do not make as much sense as the invested energy to create a better processor is really really high – so better to run the old machine until the magic smoke escapes or it is no longer able to meet your needs at which point a whole new more efficient all round machine makes sense. Where the high rated PSU should outlast the computers useful life (so be good for the next computer) and has a lower energy cost to create.
We need to make stuff more energy efficient for sure, i think this is THE Best way to “create energy”.
But, we are in a “race to the bottom” when it come to price, for parts etc. We could also be in a “race to the top” instead.
If we would, for instance, take the most efficient part of anything (psu, tv, transformer, etc etc) and set them as the standard. Then tax them 10% lower than normal, add that 10% tax to the least efficient in the same category… It would mean that either manufacturers up their game or die off. Then two years later see if there is a new “best efficiency”, give that a tax break etc etc… This could be applied to everything we buy. I mean, I cringe when I see “2000 Watt desktop speakers” knowing that they probably will only have 10% of that as output. It’s also criminal imo…
As for power production, I think we are starting to see that wind and solar are not the magic sollution, as they are unsustainable in many ways. They MIGHT be a nice transition technology to help us along to a better tech. Like THORIUM?
In the mean time windfarms at sea could create hydrogen and pump that to shore via the old gaspipes that are often still there. It’s probably the best way to store energy for when there is no wind or sun.
If they started added gallium to beer cans, then they might be onto something…
So, you could light your burps while drinking?
Nah, gallium reacts with aluminum in a manner that breaks down it’s structure faster than it can oxidize. Imagine being able to poke a hole through your can of beer with your pinky with no effort. Kinda like wet toilet paper.
Hydrogen is not a fuel, and never will be. It is an energy-storage medium. Its position on the periodic table of the elements ensures that.
No, iron is not a fuel from its position in the periodic table. Look up its nuclear binding energy. Hydrogen can be an amazing fuel, we just haven’t figured out how to fuse it easily yet.
Since H2 molecules are REALLY, REALLY tiny, I can see that it would be difficult to make fuses for them.
And how would we attach those fuses? Would we be able to tell if a fuse is blown by looking at it? Or, would we need tiny ohmmeter probes?
we know how to fuse hydrogen, first you need 0.08 solar mass of hydrogen…
hydrogen is a very terrile fusion fuel, its got a tiny cross section, that’s why stars are big. deuterium/tritium now. and i figure by the time with get to 3rd or 4th gen power reactors we will be up to aneutronics.
“hydrogen is a very terr[b]ile fusion fuel, its got a tiny cross section,”
need to precisely direct some energy at something? perhaps a laser is just what you need.
Hydrogen IS a fuel (ask the Apollo program). Fuel cells are energy storage DEVICES.
Let’s hope this is a real solution and hope it can help reduce pollution emissions. Covid is NOTHING compared to the real disaster coming with Global Warming. But, when are people going to get smart? Did you know that there is more pollution generated during the creation of a car than what that car emits throughout it’s life time???? This means we need to stop buying new cars so soon and try to get 25 or more years out of the cars we already have. Of course any car salesman will tell you I’m wrong!
The restrictions against Covid are more then enough and with all that economic breakdown only a tiny reduction in CO2 emission was measurable. I hope, that all climate hysterics get that and recognise that we must not destroy our economy even more only for hysteric fear of a hypothetic global warming.
What about Hydrogen Battery? Combustion engines have low performance.
Seems to be a drowning swat from a dying industry.
This just isn’t true… 40% efficient internal combustion engines are on the market now from Mazda and Toyota has similar. Without a hybrid system at all… that is actually a very high bar for electric cars to beat and some almost certainly don’t meet that, and any electric car fueled from the grid supplied from coal/gas probably does not met that due to the losses going from generation, to transmission , battery charging losses etc…
Mazda also has plans for it’s next generation ICE to be in the mid 50% thermal efficiency range…. at which point its a moot point, if you like gasoline engines its just as clean as electric. And if you throw in future bio fuels, it could end up actually being carbon neutral or negative.
40% LHV? Yeah, that’s pretty terrible compared to electric batteries (>90%) and fuel cells (>55%).
Electric batteries have several real-world disadvantages going against them.
1) Grid efficiency, including transmission and conversion such as chargers, is around 90%
2) The efficiency of the battery itself in discharging and power conversion, plus self-discharge, 90%
3) The manufacturing energy cost: whether you manage to use up all the possible cycles before the battery dies from old age or becomes otherwise discarded (diminished capacity).
In the ideal case, the cost to manufacture a Li-ion battery in terms of energy is about 10% of the overall lifetime stored energy, but it can be more than 99% for UPS batteries that sit on standby until they hit their shelf-life and get replaced. You never actually put much energy through them, you just keep them on trickle until they rot, so their energy efficiency is nearly zero. The actual utilization determines how efficient your battery is.
Assume a battery is good for 2,000 cycles and 10 years. If you give the EV a 200 mile range, you would have to drive about 400,000 miles to use it all up. The average driver will do about 150,000 mi so they’re only using about 38% of the potential cycles. The manufacturing costs therefore grow from 10% to 27%, which means the efficiency drops from 90% to 73%. You can give different numbers, for this, like suppose the best cost is 5% and the battery lasts 4,000 cycles. Well, since the ideal cost is only obtained at full utilization, you don’t get 95% efficiency but again the same 73% because you won’t drive any more than you do.
Count them all, and what do you have: 90% x 90% x 73% = 59%
And that’s assuming that the power stations are all 100% efficient, clean and renewable, and that you don’t need things like grid batteries for buffer, so you don’t account for their losses. There’s still more, such as standby losses in EVs that are constantly on to remain online for software updates, or the need to heat up hundreds of pounds of batteries to charge in the winter… etc.
When you count all the little streams, it becomes a big river of wasted energy.
Besides, with the renewable economy we’re trying to have, we are facing a situation where we have to start stocking months and months worth of energy to balance out the monthly and yearly differences in energy production – we can no longer draw energy from the ground as needed. The current strategic reserves last 2-3 months, so that’s the minimum standard. Electric batteries will get us 2-3 minutes on the grid scale.
The only economically viable way to do that is to start making synthetic gas: hydrogen, methane, and various heavier hydrocarbons to ease up the storage requirements, and these stockpiles have to be topped up some years, other years not, so there’s plenty of idle production capacity to make synthetic fuel.
When you have that system in place, it is no longer sensible to convert it back to electricity at a great loss – you get far better use out of it in engines that can burn the fuel directly – even in traditional gas engines, but fuel cell hybrids would obviously beat battery electric cars 10-0.
Well, we could cut the transmission costs (inefficiency) of electricity by driving to the power plant and plugging the car in there when it needs to be recharged!
(I also have plans for a sailboat that uses an electric fan to push the sails, the electric fan is powered by a wind gen.)
“The only economically viable way to do that is to start making synthetic gas:”
You can store energy by pumping water up a hill. I’m not sure why you’d make synthetic gas when you can collect net positive energy when making the right kind of biogas. (input energy + energy from sun)
>You can store energy by pumping water up a hill.
Pumped hydro storage is 80% efficient.
Please calculate how much you need to pump to store 1 TWh. The compare that to the fact that the EU gas grid already holds about 200-300 TWh worth of gas.
Of course if you can make biogas, that’s a bonus, but that’s basically a very inefficient solar panel since you’re throwing agricultural product at it. There’s not that much waste around. You can collect much more energy per square km with solar panels, and much more efficiently.
40% efficiency for an ICE is from raw fuel to kinetic energy. His point is that although electric motors are well over 90% efficient, what do they get recharged from? A regular single cycle fossil fuelled generating station is 30% efficient (most of the heat gets wasted into a lake or cooling tower). Electric high voltage transmission lines lose 5%, the distribution system that feeds your home loses 7% and the battery charger probably loses another few percent. So from fuel to electricity into the car battery is only about 25% efficient. So your electric car is 25% efficient raw fuel to kinetic energy.
Combined cycle natural gas electric generating stations are 50% efficient so that improves it to about 40% overall, like the efficient ICEs. But best would be to use 100% renewable energy to supply the electricity (solar, wind, hydroelectric). However, the electricity delivered to your home is a blend of sources and renewables (in the US) are a small percentage of the total.
Were you under the impression that fuel gets to the vehicle’s tank with zero waste? Sourcing, pumping, refining, transportation all consume vast quantities of energy. By some accounts the energy to refine a gallon of gasoline is greater then the energy require to push an EV the same distance that the gasoline would. All while the oil is still left there in the ground.
>Were you under the impression that fuel gets to the vehicle’s tank with zero waste?
It’s actually much more efficient than the electric infrastructure. Transmitting fuel by truck over a thousand miles loses a negligible portion of it to run the truck, while an electric transmission line loses 5-10%.
> By some accounts the energy to refine a gallon of gasoline is greater then the energy require to push an EV
That depends on how you count. Petroleum refining produces other products at the same time, but it’s generally estimated at 80% efficient. A gallon of gasoline would lose 7 kWh which gets your Tesla 28 miles.
To be fair you should also consider the wasted energy in refining and transporting fuel, extracting oil, etc.
You beat me to it. Also the endless dead from our wars to “secure access to this vital resource”.
How much of this vital resource would the dead have consumed were they not dead?
“How much of this vital resource would the dead have consumed were they not dead?”
Not really, fewer people would have been born as a consequence of living in peaceful times. It’s a huge waste of resources to feed and educate a person to age 20 only to have them die in an explosion. Implying their death is a net positive is a bit misleading considering that we could have used that person’s short life to produce something instead of for killing.
The well-to-wheel efficiency of a car running on petrol (in terms of CO2 expelled) is about half of that of a similar electric car “fueled” from an oil-fed power plant. Just because electric motors are so damn efficient.
Given the abysmal build quality of Mazda cars, i wouldnt hold my breath regarding anything they claim.
Big problem is that this is unique in various ways, and to become a success you not only need to make/adapt the cars, but also set up a production plant, and distribution infrastructure.
Yeah, I see the oil companies buying up his patent just to keep it off the market.
B^)
This is right up there with HHO on the BS meter….
I can’t even believe this made it onto hackaday…. its literally a scam that has been making the rounds since 2008 or so. If it was real and or practical it would already be in use. The fact is no solid fuel system is ever going to overtake liquid fuels unless it is something crazy like Thorium fuel cells you replace once a year or something like that. Otherwise the logistics are just terrible…
Also this system doesn’t use the split water as the primary fuel… its just for idling etc… complete nonsense.
That’s the standard process of making aluminium: the Hall-Héroult process [1]. And it does burn carbon into carbon dioxide, apart of eating electricity for breakfast.
IMHO this is just a very inefficient kind of battery. And then the H2 is burnt in an internal combustion engine, multiplying the efficiency by another factor 0.5..0.6.
Not impressed, sorry.
[1] https://en.wikipedia.org/wiki/Hall%E2%80%93H%C3%A9roult_process
YES, even with the greatly exeggerated efficiency of 0,5 or 0,6 :-) Show me a paractical ICE with that high efficiency :-)
Not an expert, but I believe even with a hydro powered aluminum smelter, there is still a large quantity of CO2 produced as they use carbon electrodes that grab the “liberated” oxygen and produce CO2.
https://aluminiuminsider.com/leaders-emerge-in-the-aluminium-industrys-race-to-zero-carbon/
Yes, but in the article:
“Alcoa Corporation, Apple Inc., and Rio Tinto Group teamed up on a breakthrough process that emits pure oxygen rather than carbon dioxide, eliminating the latter entirely from the production equation. The process, which was formed under the aegis of a joint venture named Elysis, utilizes a proprietary metal to form the anode used in smelting, replacing the carbon currently used for the purpose, and therefore removing the element from the chemical equation entirely.”
“Proprietary metal” is basically copper.
What about hydrogen embrittlement of the engine?
Most have been solved coating, and your car would be worn out before it became a issue. Hydrogen wants to escape it has always been a big problem.
A good question. I don’t think it will have effect on the engine – as I understand it its a very very slow process that requires long term exposure to H. In the engine that will never happen as its either burning or burnt all the time. But I’d like to see a study that looks at this I could be wrong.
Think of it as a way to make electolisized hydrogen portable in the form of aluminum and water instead a high pressure vessel. If you compare bottles of H2 and the aluminum method, the relative efficiency isn’t so bad. On the other hand, if you get your hydrogen from natural gas, the efficiency of the aluminum method is not so great. Maybe if you include the cost in power to compress the gas and to haul around high pressure tanks, it gets better.
No. Even on paper hydrogen sucks as a fuel source for ICEs. Low energy density and tons of NOx emissions.
You know most modern cars have systems to remove NOX, they are called for one a catalytic converter, Additional modern engine management systems keep cars in tune and tell you when you have a issue. You need to work better on your quips also this hydrogen is produced in the car and the pucks are made from waste energy. New process aluminum does not use carbon electrodes. Great transition tech for us and the third world. Works in fuel cells also, bang!
But, won’t urban dwellers be happier with more Nitrous Oxide in the air?
It’s NOx not nitrous oxide. The x stands for all the various amounts of oxygen.
Ah! I stand corrected!
Thanks!
Hydrogen is not gaining ground. Companies building HFC vehicles are abandoning the technology and the sales ratio of HFC’s to BEVs is going down. The primary reason why HFCs will fail is that they are a more indirect means of capturing renewable energy (i.e. because if you don’t want to use fossil fuels, you need electricity to power the generation of hydrogen and compress it), and therefore far less efficient. Ergo, using HFCs will slow decarbonisation.
There’s a good, long blog series about why HFCs aren’t the way forward.
https://ssj3gohan.tweakblogs.net/blog/11470/why-fuel-cell-cars-dont-work-part-1
Yes, this solves (or at least improves) just one of the problems with hydrogen cars. The efficiency is probably even lower than the efficiency of hydrogen cars using pressurized hydrogen.
Insisting on batteries will slow the adoption of renewable energy technologies, since they’re too expensive to store enough of it, and the rising demand will keep them from getting any cheaper. Countries are hitting ceilings after about 20% share (not counting hydro), because they can’t put it anywhere. So far they’ve been selling it across borders to get rid of it and paying curtailment to shut the turbines and solar farms down occasionally, but when their neighbors are doing the same thing and the curtailment costs are rising, the problem comes back.
Using synthetic gas/fuel (power-to-gas) allows massive stockpiles of renewable energy at modest costs, and the infrastructure to store and transport it already exists.
The infrastructure to use the fuel also exists: conventional engines can run on it without the lossy conversion to electricity.
Why not do more in the way of thermal storage for the loads that are thermal in nature – HVAC, hot water, and refrigeration? Those happen to be the majority of residential energy use (and a significant percentage of commercial energy use) so on site thermal storage makes a lot of sense. Some of it, like hot water and freezers, can have thermal storage added at close to no cost by merely adjusting the setpoints based on availability of surplus energy.
> after 20% share, because they can’t put it anywhere.
This actually is economics , not any kind of technical issue. Industry uses vast amounts of energy as (often low grade) process heat, and can use as much excess electricity as you can make, for heating.
It doesn’t want to , because fossil fuels are very cheap in bulk, and using electricity means capital investment it would prefer not to make unless forced to.
As an example here in NZ, dairy companies are using forestry waste biomass to dry milk powder, instead of electricity, while steel and aluminium manufacture _must_ use carbon.
This is a mis-allocation as the biomass could eliminate a hard carbon requirement if used in the right place – which is never process heat.
It is also true that until you have stopped burning all carbon for heat, you don’t really need** to transition cars, trucks, airplanes etc to electricity .
** the improved effiicency of electric vehicles (and air polution) makes it somewhat more worthwhile.
This is also why inefficient hydrogen cycles like this one, are really pointless and counter productive. (until there is a big excess of zero carbon electricity)
The HFC was pretty much a boondoggle to begin with – just a way to appear to be doing something for PR back in 2000’s. It’s an eternal research grant generator that never really goes anywhere.
SOFCs can burn any fuel you can vaporize, including straight up coal dust – so they would behave like regular vehicles.
This fails on so many levels, but the elephant in the room is:
Why is the water and the aluminum fuel button getting hot?
Answer: MOST of the energy contained in that aluminum button gets wasted in the reaction to produce aluminum oxide from the water. Although each button reacts with only about its own mass in water, they use ten times the amount of water required so it doesn’t all boil away: there’s more than enough energy in that reaction to melt all the aluminum. Only about a third of the energy embodied in that aluminum comes out in the form of hydrogen.
They’d get more energy out of the aluminum fuel by using the steam from the reaction and driving the engine pistons directly, rather than burn just the hydrogen waste product.
Too bad you couldn’t do both, and burn the hydrogen-rich steam. I suppose you could add a second engine (or a fuel cell) running off the hydrogen exhaust of the steam engine.
I’m just musing here Paula,
but will “stealing” the heat from the reaction to make steam, decrease the efficiency of the reaction?
I’m pretty rusty on reaction coefficients, but I’d guess this one pushes so far to the right it won’t make a significant difference at any temperature and pressure you’re able to keep inside any reasonable container.
From reading another article, the process of recycling the aluminum, is to use waste power provided by solar and wind and hydro, the galium never goes away, and use this tech in addition for a latter storage of energy to be reclaimed as power or transit cuts waste immensely, it works well as a transition tech, i.e in the third world you would be waiting a long time for first world tesla parts and service, while those lions circle your truck. Where in old tech your guide climbed on the hood opened it and fixed the fuel line.
I like it, a lot of thought has been put into the tech. It is a great way for old tech to be used gracefully, i.e. until we can come up with something better, and awesome way to store lost power long term.
Also Nox? come on guys we have a multitude of ways of reducing and eliminating. This is darn awesome tech, also remember batteries store nothing, when they are full and slowly discharge. No one loss until used, no oil refined pucks can be made anywhere, can be used for conventional and fuel cell! And no high pressurized tanks!
Also to naysayer who say this has been making the rounds or thorium reactors. Research is a on going process. Want Thorium reactor move to india, it is not a common element in North or South America, India has a lot of it.
I would not mind working for them, pretty coolio. And I have not said that about anyone in over a year!
I’ve seen a system like this before, in which a relatively small amount of hydrogen was just added to the gasoline being injected. The justification in that system was not that the hydrogen provided any significant power, itself; (because it was being made by electrolysis powered by the alternator) but because it improved the gasoline burn by reducing the “flame quench”. My understanding of this is that the burn of the gasoline is limited near the wall of the cylinder because, as hot as the cylinder is, it’s still cold enough to stop the combustion in its immediate vicinity. The claim was that the hydrogen reduced region or shell around the cylinder with unburnt gasoline.
The mean free path of molecules in air at atmospheric pressure is a small fraction of a micron. Under compression in a cylinder it’s only going to be a few nanometers. I can’t imagine the hydrogen is going to do diddly squat to reduce the unburnt shell to any significant extent.
Carbon free aluminum process for those that say carbon is released and yes currently but is in decline. https://www.reuters.com/article/us-apple-aluminum/apple-buys-first-ever-carbon-free-aluminum-from-alcoa-rio-tinto-venture-idUSKBN1Y91RQ
I think I’ll stick to LPG for the time being…
Technological ‘solutions’ are never ‘green’.
How much land was destroyed to mine the ore? How much fossil fuel burned to mine, process, and transport materials around?
Aluminum is worse than steel for energy required to process.
Of course at the the end everything consumes energy and ressources. That does not mean, that this aluminium fuel makes any sense. But on the other hand I do not want to give up my livestyle with a car, computers and other ‘luxury’ things.
Can you run an engine on acetylene? I’ll bet calcium carbide is cheaper than this alloy.
Acetylene has a significantly worse record of exploding than hydrogen, which is saying something. At 1.5 atmospheres, acetylene autoignites, so you have to be pretty careful. It’s also much harder to burn cleanly than hydrogen, or gasoline, or diesel, or just about anything else: it tends strongly towards unburned hydrocarbons and even unsaturated hydrocarbons.
Plus based on their respective gibbs free energies, you’d have to carry about 20x as much calcium carbide by molar ratios to get the same energy, which would work out to about 25 times the volume.
That’s the draw of aluminum: it has incredible energy density if you can just figure out how to efficiently harvest it.
When I was a kid people were still using carbide lanterns in caving, and man I saw so many of those things burst into uncontrolled flameouts because someone messed up the water feed into the carbide. Nothing quite like being in total darkness, having a giant fireball erupt from someone’s head, and then trying to deal with someone with face burns… in the dark, a three hour rock climb back to the outside world where the ambulance would be waiting once someone got out there to call for help. Sure, this is my bias speaking, but it’s really hard for me to think of anywhere that acetylene is a really good idea, including welding.
I caved with carbide extensively, and never saw any of the problems you recount.
If there were face burns then I can only assume that it was a cap-mounted “Stinkie”?
The waist-mount remote generators were 100% safe in my experience (Petzl Ariane, Malham, random Russian titanium thing). Not 100% reliable, but a warm, friendly bubbling friend in a cold place :-)
I still think that carbide is by far the nicest light to cave by, you can look around with your eyeballs rather than you neck.
But it has no place in automotive engines.
I have a carbide headlight on one of my motorbikes though :-)
But Calcium Carbide is just an indirect and inefficient way to burn coal. The idea is to reduce the use of coal or fossil fuels alltogether.
Call me when it runs on squashed beer cans. That will be a win.
One problem, well a few come to mind, I still have to admit there are a lot of positives though.
~8% of the earths crust by weight is Aluminium (big plus)
Unfortunately Aluminium is extremely reactive so only exists as minerals (~200 to ~300 different compounds)
To extract pure Aluminium you need to chemically process and melt a compound with a low melting point (The primary commercial source is bauxite) and then apply high energy for electrolysis using the Hall–Héroult process (960 °C ;1,760 °F), the denser liquid Aluminium sinks to the bottom of the cell where it can be poured off. And large amounts of carbon dioxide are produced at the carbon anode (which can be captured).
The best Hall–Héroult process cells use about 13 kilowatt hours 46.8 MJ (megajoules) of energy to produce 1 kilogram of 99.5–99.8% pure Aluminium.
The main problem with the above is that the end product produced will be Alumina (AKA aluminium oxide AKA aluminum oxide AKA the mineral corundum). Which is not used as a raw maternal to produce Aluminium because of its high melting point (2072 °C ;3762 °F; 2345 K) and resistance to chemicals. So the world’s supply of Aluminium which is close to 100% recycled due the extreme cost of producing new Aluminium would be converted into a mineral that we do not extract Aluminium.
For ~50 MJ of energy about 1kg of Aluminium is produced, and I’m going to guess that the amount of energy released by burning the hydrogen produced from this process in the order of about ~13 kJ. Lots of good points but far more bad points. My back of the envelope maths would make me do a U-turn.
Nothing can really replace fossil fuels. Fossil fuels are created using very low energy, but over very long periods of time. But we burn it faster than we can create new.
If we want to shorten the creation time of a fuel, we must increase the amount of energy to create that fuel. Law of nature, can’t get more energy out of something than was put into it.
Now, we can put in our own energy, for instance electricity, like with these aluminium pucks. Or we can put in other’s energy, namely slave work (bacteria, etc.). :) And I think the second option will always be economically more viable.
All that we consider fossil fuel today was created during the Carboniferous Period (360 to 286 million years ago) because there was nothing that could break down lignin. So all that carbon was eventually burred, isolated and slow cooked for nearly 300 million years. Evolution happened, as it does, to fungi and bacteria towards the end of the Carboniferous Period and some could eventually breakdown lignin as long at there was more than 5% oxygen available. So creating new Fossil fuels is never going to happen in the same way again unless the oxygen level in the atmosphere drops below 5%, well maybe – never say never :)
I agree with everything else you said, but the specific events that created so much fossil fuel ended once fungi and bacteria were able to cause lignin to decay, otherwise today we would have multiple layers of oil and coal at different stages of being ready.
Essentially all energy comes from 1 of 2 sources. Nuclear fusion, from the sun, and Nuclear fission, from heavy radioactive isotopes. Everything else is just storage.
You are wrong about the aluminium oxide.
It is the direct input into the electrolysis of aluminium. Bauxit gets processed to aluminium hydroxide which is then converted to Al2O3 by heating. The ‘secret’ is the use of cryolith to lower the melting point – the Al2O3 gets more or less dissolved in it. Then you can electrolyse it.
Of course it can be extracted, but that process costs more than using fresh bauxite.
~8% of the earths crust by weight is Aluminium (big plus)
What a misleading and ultimately meaningless observation.
No future.
This is a battery with extra steps that wastes gallium.
Is it really a 20% CO2 reduction? Smelting aluminum is ridiculously carbon intensive.
From bumper to bumper sure. Well to wheel it probably burns more
This article kinda misses the entire problem with typical hydrogen cars as well – if you leave one in your car for a week, it will be empty when you get back to it. In order to make hydrogen cars have any kind of range, they need a *lot* of hydrogen. In order to compress that hydrogen to make it transportable, it needs to by cold. But, to *keep* it cold, you have to keep refrigerating it, which costs power. So hydrogen cars would constantly be draining electricity to keep them fueled. In practice, adding a refrigeration unit would be too much weight – so they manage the system the only other way they can – by constantly bleeding off the excess pressure caused by the temperature raise, while the car is at idle.
The upshot of which is – if you fill your hydrogen car up on the way home, then leave it on your drive for a week, it’ll be completely empty again by the time you get back. So all designs have to be battery-equipped hybrids anyway, so you can drive your now 10-mile-range-electric-car to a fuel station when it boils itself dry. Related note, we need to replace EVERY SINGLE FUEL STATION with cryogenic storage and delivery technology as well. Oh, and in the UK a hydrogen car costs literally double a mid-capacity electric car, or slightly more than a Tesla. And there are only two models, compared to… twenty or so electric models, and five to ten PHEV’s of the gasoline based variety?
Hydrogen cars are a dead technology which are never going to take off. The future is electric, with a less stupid energy mix powering them. As others have well explained, while the concept would solve the issues that I describe above (which are the only things it does theoretically benefit), this is not the panacea to save them.
I don’t think that hydrogen powered cars are a useful way to go.
BUT:
1. I think you mix up pressure storage and cryogenic storage
2. The idea of this – overall incredibly inmefficient – process is to AVOID H2 storage. If you read the article, it is about creating the H2 in situ on demand.
I can imagine cryogenic hydrogen for long distance trucks. They are just too expensive to stand for a week and need too much energy for battery operation. For cars I think battery operation will be the way to go.
Exactly, much like milk floats worked well as purely electric vehicles for decades before this mainstreaming of the idea back when batteries weight was most likely measured in tonnes.
The right fuel for the right type of vehicle helps hugely, so hydrogen cars probably do have a place in the world – taxi, bus, the traveling tradesman etc will be doing the mileage often enough to make gaseous hydrogen more viable for them than batteries (assuming the infrastructure is built – but LPG is pretty common and similar in engineering so no reason it won’t be). But your short daily commute to work or the shops probably battery or hybrid.
The real application screaming for hydrogen to me is Aircraft, the big long haul aircraft all run from a small number of airports so the infrastructure to change fuel types is relatively cheap. If Aircraft can be electric motor and fuel cell they don’t even need to generate high altitude NOx etc, they really could just produce water as a waste product.
“milk floats”
I have heard of “root beer floats”, but this is the first time I’ve heard/read of “milk floats”!
B^)
Comparing the industrial use of LPG to the commercial and residential use of compressed hydrogen is dishonest at best
Wonder if you could get rid of the use of Gallium by applying some nanotechnology to the Aluminium.
I guess the Gallium is a whetting agent to kickstart the oxidization process and keep it going. The problem with oxide is that it can’t oxidize twice. ;) So once you have an oxidized layer on your aluminium, the water can’t reach it anymore and the oxidization process stops. That’s why you need some catalyst/whetting agent.
What if you could structure the aluminium molecules in such a way that no layer of oxidized aluminium will be formed? That during the oxidization, always some new non-oxidized aluminium gets exposed?
Actually, maybe that would lead to such fast oxidization and creation of H2, that the sudden expansion of H2 might already cause an explosion of the container.
I don’t know, just brainstorming without having much knowledge of this topic. :)
You need the oxide layer for the Aluminium to be stable in air anyway and “applying some nanotechnology” sounds also expensive. You can also use lye (NaOH) to break the oxide barrier and let the Al react with H2O But I think the advantage of the gallium is, that you can get it out of the reaction more easily. It is a catalyst, not a consumable.
Sounds like the making of a good movie, I’ll name it “Back to the Future”
Henry Ford had several hydrogen only cars, and the way they stored hydrogen was interesting.
No high pressure vessels, rather his design used many pounds of fine steel wool. Hydrogen would be absorbed into the metal at ambient temperatures, and when heated, the gas is liberated.
Heat to release the gas came from the exhaust system of the vehicle.
Hydrogen gas would reach a pressure equilibrium dependent on the temperature of the steel wool, the hotter the steel got, the more hydrogen would be released.
The limiting factor was the steel wool becoming brittle and falling apart after some time.
So this was an early metal hydride storage. I think with modern storage alloys you can store about 6% by weight of H2 – not very much.
Now the payback time for solar panels is only a few years. ‘Moore’s law’ does not come from Micheal Moore :-)
But energy storage is stil la problem on the necessary scale.
Does that maths add up?
Google suggests that a number of factories in China create around 3GW of panels per year.
If a single 300W panel makes the equivalent of 2 hours full power a day (ie about 1/12 of rated power to allow for night, seasons, degradation) for 10 years that’s 2MWh per panel.
If the factory makes 3GW / year then that is about 10 million 300W panels per year, or about 2000 panels per hour for a 12 hour day.
So the factory makes 2MWh x 2000 panels / hour = 4 GW.
ie, for the stated calculation to be true, the factory has to consume more than 4GW of power on average.
The largest power station in the world (also in China) is the Three Gorges Dam, as 22GW total output.
It doesn’t seem likely that a single PV factory would consume 4GW of power.
What about the mining/transportation costs of the raw materials/finished product (i.e. well to wheel)
Does this Chicom factory also do the silicon refining?
It really doesn’t. You can make it add up if you add in caveats on the panels having tiny lifespans,rubbish orientation etc. But even the earliest generations of solarPV while degraded tend to still produce meaningful power and we have learned enough to prevent degradation quite successfully now so modern panels will probably still work well enough long after we are all dead. Making those arguments bovine excrement used as an excuse/justification for those that make on the current set up.
The carbon payback time isn’t anywhere near that bad – but very variable depending on where you source your panel. China is the worst place to get them as their power grid is mostly very dirty coal but still only a handful of years to payback (Can’t really be precise there as if you have Sun tracking and perfect location you can get near the panels rated maximum through many hours on most days and probably be paid off in first years summer months, just a fixed mount but in a good location will be more like a couple of years, and fixed mount in a really crap location will still almost certainly produce some power but payback could then be outside a fair lifetime of the panel).
Financial payback is more of an issue and a very complex one, but investment in solarPV on your own property isn’t going to make you rich. Have not had a system here that long yet but looking like less than three years just on the power bill saving. In my case through the summer only take on a tiny bit of grid power over night (not much battery store) and even on a bad day they tend to keep the freezers running during daylight hours at a minimum. So I probably could go off-grid entirely if I wanted (with investment in more storage) it would just take some degree of power management – don’t run the dishwasher, washing machine etc all at once on the bad days – spread it out over the week or do it all on that sunny day when the stores will fill no matter how hard you try to use it all. I definitely do not advocate off-grid if you can be on the grid though – even if you are wanting to live in off-grid sytle being connected gives you security, and when your stores are full any excess power you generate can still be used usefully!
The real issue with SolarPV is the energy storage with batteries you end up manufacturing them all the time with, Hydro and CAES are better on that score being simple mechanicals that can be cheaply kept running but need the right location, are larger etc. A simple tank full of hydrocarbons represents a significant energy investment to make (much of it happening over the eons since Mr Dino got buried and if you add in that the invested energy in a tank of petrol vs its chemical potential is hilariously bad), but that fuel is still going to burn just fine after long term storage. But that doesn’t change the cost payback time on the panel – it just changes how you have to use and store energy.
The only thing 100% correct in AF6LJ’s post is how wasteful it is to keep converting types of energy, which is something we are doing regardless of where the energy comes from. It is also something we have to do sometimes as it is more efficient or safer to do so, but worth paying attention to and trying to reduce where practical!
Seems like you didnt actually do any math there at all, you just made up some numbers that worked
Wow that’s a LOT of assumptions and a LOT of factors being ignored. How are materials getting to the factories, zero emission faries??
Yes, it was very much an estimate to see if the numbers add up at all.
There are lots of assumptions, aimed at getting an order-of-magnitude feel for the plausibility of the original assertion.
Let’s try another. back-of-an-envelope calculation to put the 4GW power figure in context.
The worlds biggest aluminium smelter makes 1 million tons per year, and uses about 15kWh per kg to do so.
So, that’s 15×10^12 Wh/year = 1.7GW of power.
Is the world’s largest solar panel plant _twice_ as energy intensive as the worlds biggest aluminium smelter?
“Here’s the best part: the oxygen binds with aluminium and becomes aluminium oxide powder, which can be turned into new pucks indefinitely with the addition of more gallium.”
That’s ‘with the addition of more gallium…and energy’ for those who might be wondering.
“Each day, the trucks will start out running on hydrogen and automatically switch over to gas when all the canisters full of alloy pucks are spent”
I’m late to the party, so the video won’t load for me. Can you clarify ‘switch over to gas’. Do you mean switch over to gasoline (petroleum) or switch over to gas (LPG)?