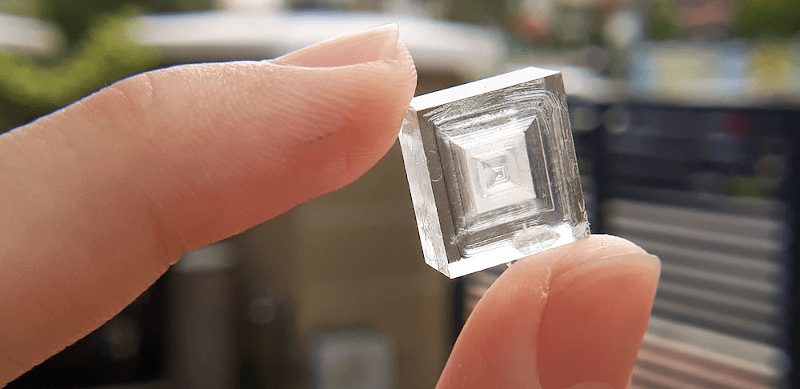
[Chase’s] post titled “How to Grow Sodium Chloride Crystals at Home” might as well be called “Everything You Always Wanted to Know about Salt Crystals (but Were Afraid to Ask).” We aren’t sure what the purpose of having transparent NaCl crystals are, but we have to admit, they look awfully cool.
Sodium chloride, of course, is just ordinary table salt. If the post were simply about growing random ugly crystals, we’d probably have passed over it. But these crystals — some of them pretty large — look like artisan pieces of glasswork. [Chase] reports that growing crystals looks easy, but growing attractive crystals can be hard because of temperature, dust, and other factors.
You probably have most of what you need. Table salt that doesn’t include iodine, a post, a spoon, a funnel, filter paper, and some containers. You’ll probably want tweezers, too. The cooling rate seems to be very important. There are pictures of what perfect seed crystals look like and what happens when the crystals form too fast. Quite a difference! Once you find a perfectly square and transparent seed crystal, you can use it to make bigger ones.
After the initial instructions, there is roughly half the post devoted to topics like the effect growth rate has on the crystal along with many pictures. There are also notes on how to form the crystals into interesting shapes like stars and pyramids. You can also see what happens if you use iodized salt.
If salt is too tame for you, try tin. Or opt for copper, if you prefer that.















There are a host of interesting water soluble compounds that a person can get busy with growing crystals, so you don’t need to fool around with furnaces full of molten metal. Copper Sulphate is a great one, although toxic (as are many metal salts).
Sodium Chloride is a bit unusual in that not much more dissolves in hot water than in cold, so methods that involve oversaturating solutions by heating, dissolving to saturation, then cooling are not very useful. Good old evaporation is
your friend.
Oh, and there are no ugly crystals, though some are more attractive than others.
Do it with potassium chloride, and you could make your own IR optics – if the meed took you.
Mood. Too much mead. Sorry.
I also wonder about making Rochelle salt crystals, since these are piezoelectric, and calcium carbonate, which if made as nicely as these salt crystals, forms calcite with its characteristic image splitting characteristic.
Esentially, you cook bicarbonate of soda and then add cream of tartar.
If you buy the bicarbonate of soda as a cleaning compound, not only does it usually come in larger quantities but it’s also usually of higher purity than the food grade option.
Cream of tartar is getting trickier to find these days. [ As is borax cleaner, side note, good for making bouncy slime. ] but both are still around, if you know where to look.
One of my favorite crystals to grow is probably Aluminum Potassium Sulphate. [ crystal deodorant ] They have a similarly interesting structure, but a different resultant shape and usually grow large pretty easily, given a saturated solution and a little evaporation time. But you can even viably grow tin crystals at home, if you utilize some electrolysis.
I brought the (cooking grade) ingredients to try do just this once, sadly never go round to it.
I am a bit lazy, and it’s a lot easier to have ideas than actually do them, it’s my biggest failing…
So long as you pick some of the best ideas and do them! Just think, some people never have any ideas.
Some lazy people became very rich, by finding an easier way to do something!
You just described my life. Well, let’s just fist bump and move on.
Thanks Al, I’ve never seen home-grown crystals this perfect!
These are really nice, might be fun to mount one in a 3D printed ring.
Don’t forget to lacquer the crystal, so it’s not ruined by moisture in the air.
I see what you did with the title. Last weekend I saw The Crystal Method perform at the Baltimore Soundstage. It was a great time!
They do the music to PS1 game N2O. We have two copies ‘cos the game is awesome withe the music – best PS1 game [in my humble opinion].
I’m sure it seems odd but they’re one of my most frequent playlists. I have pretty wide-ranging musical tastes lol
Why do people always play with such dangerous chemicals? Sodium chloride is really nasty, it does only dissolve in DHMO, which is exactly the stuff they put in vaccines. Stay safe, keep it organic.
Copper(II) sulfate pentahydrate is available as root killer at farm co-ops and at some Lowes, Menards, etc. That’s if you want to make beautiful blue crystals.
Bismuth crystals from Pepto-Bismol Antacid Tablets.
Rochelle salt is also piezoelectric, and pyroelectric.
Scuttles off to try and build a homemade X-ray generator using this effect, and a capped syringe with a Peltier at one end that holds the crystal so it can be heated and cooled.
Alternate plan is to use a modified pyroelectric security sensor.
The best method would be to Epoxy everything in place once its working.
In the olden days liquid metal such as Hg was used to draw a vacuum but these days other options like BiInSn exist.
For extra bonus points use electrostatics to further evacuate the chamber.
FYI, the cheapest source of non-iodized sodium chloride that I found was water softener salt, which is often sold in big box stores for less than $10 for 50 lbs. I got sick of paying twice that much for a few ounces of “Aquarium Salt”.
Salt windows and lenses and prisms are used in IR instruments like spectrophotometers. It is a lot cheaper than sapphire. I think they are made with pressure, not crystals but not sure. https://www.bucksci.com/products/nacl-13x2mm-disc-500a1
Got no use for such lenses but I am amazed that you can buy lenses made of table salt.
Well done! For a moment I read “DHMO” as “DMSO” and thought “WTF”? Then I realized you were talking about H2O, which is (not) oceans away from NaCl!
Are these crystals, essentially rocks? Could I fire them ground on pottery? I’m thinking so! Please send mix to test
1) Yes! These are rocks!
2) ..rocks that dissolve in water.
I have no idea whether they would make it through a day in the kiln. Ionic compounds are pretty hard, in general, but then, most glazes are ionic compounds. And it doesn’t take a very hot flame to turn sodium chloride into a blaze of yellow, so while it may be worth a try, maybe try it with crystals that aren’t your favorites.