What if there was a job where you built, serviced, and prepared science demonstrations? This means showing off everything from principles of physics, to electronic theory, to chemistry and biology. Would you grab onto that job with both hands and never let go? That was my reaction when I met [Dan Rosenberg] who is a Science Lecture Demonstrator at Harvard University. He gave me a tour of the Science Center, as well as a behind the scenes look at some of the apparatus he works with and has built.
Electronics and Physics Demo Gear
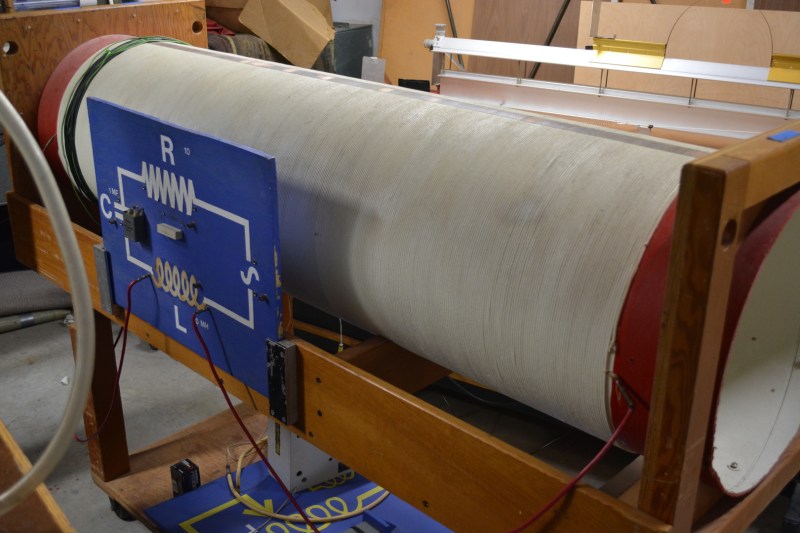
Here is a great example of what a physical demo for electronic principles is all about. When it comes time to explain inductors, [Dan] rolls this baby out for the professors and makes sure it’s ready to go in time for class. The giant pipe is wrapped with wire and connects to the schematic board on the front of the rack.
Of course it’s not just electronics that are covered in Harvard’s Science Center. Here is a microwave demonstration that uses this large black horn to generate the waves. The small incandescent light bulb is held in some plastic tubing on the end of a stick. Insert it into the path of the radio waves and it lights up. We wonder if your hand will feel hot if you don’t keep it out of the line of fire?
 Speaking of waves, stored right next to the inductor demonstration is a wave table that projects the patterns onto a screen. This is a really clever setup that predates the proliferation of document cameras in classrooms.
Speaking of waves, stored right next to the inductor demonstration is a wave table that projects the patterns onto a screen. This is a really clever setup that predates the proliferation of document cameras in classrooms.
You can make out a plate of glass laying flat. Just to the right and above is an audio speaker mount to face down at the plate of glass. To use the apparatus you pool water on top of the glass, and connect the speaker to a signal generator. Light shines up from below the glass, and reflects the wave patterns in the water on mirror which is mounted at a 45-degree angle to project the image onto the back of the white screen. I remember seeing these wave demonstrations on a reel-to-reel film in High School physics class. I think the apparatus easily doubles the cool-factor in this demo compared to a pre-recorded video.
For standing waves and wavelengths there is a long, clear oil-filled tube. A speaker at one end produces the waves which, when tuned to the correct wavelength produce a very visible standing wave. There are fluorescent tubes built-in to make the oil easily visible.
Dan posed next to the really big meter for scale. Again you can easily get a headless meter and project its output on a screen but you have to admit that this just feels more awesome. I think the same about demonstrating fire tornadoes and locking a freshman in a Faraday cage as the electricity storm (demo) rages around them.
This was really just the tip of the iceberg. The storage rooms are lined with cabinets full of demos, and every bit of floorspace is occupied by larger builds or collections like this pile of circuit illustration boards.
Because this was an impromptu tour I was only able to take pictures of the areas for which [Dan] is responsible. But we did get to walk through the chemistry and biology staging areas. In addition to these physical exhibits, the Demonstration Services are responsible for preparing chemicals for those demos and cleaning up afterward. There was even a 7-foot tall double-helix of a bit of human DNA that was about 2-feet in diameter. [Dan] assembled this from a kit with thousands of parts. He used the Tom Sawyer method of doing the tedious work in the lunch area — attracting students like flies who were eager to help.
Building these Demos
If you want to build great stuff you need a shop and tools. [Dan] and a couple of his colleagues have a small bit of office space, but hop through one door and enter this sizable workshop. There are a ton of awesome machine tools; somehow I only snapped a picture of one-half of the shop, there is another area behind me with the rest of the gear.
As with any hacker, [Dan] likes to keep a well-populated junk bin. Shelves around this area are decorated with art made from the leavings. I really enjoyed the sculptures made of old motor commutators. It’s also hard to miss the stacks of hard drive magnets adorning many of the metal surfaces. There are hundreds of them and [Dan’s] sure he will find a really cool use for them some day.
First Programmable Computer in the US
A big bonus on this trip is the huge computer installed in the building’s lobby. This is the Harvard Mark I, the first programmable computer built in the United States. The machine takes a program on the input tape, along with the positions of a huge settings register, and automatically calculates an output. It was used during the Manhattan Project to calculate implosion models used when developing Trinity, the first atomic bomb.
This Chain is a Lie and Demonstration Services Videos

It’s always fun to throw a bit of a riddle into the tour. In the same part of the lobby as the Mark I (seen here from behind) there is a sculpture of a molecule, along with its makeup labeled on the stairway behind it. [Dan] tells me “the chain is a lie” but my chemistry chops are lacking so I couldn’t work it out myself. Can you spot the problem? If so, leave your explanation in the comments below.
If you find these Science Lecture Demonstrations as fascinating as I do, you’ll want to grind through their YouTube channel. I picked out two of my favorites below. The first features [Dan] showing the paramagnatism of Oxygen. The second is a blast from my childhood; a fission demonstration using mousetraps and ping pong balls that I first remember seeing at a very young age on the Mr. Wizard’s World television program.
Now if that isn’t even enough for you, all of the demos are cataloged on the Demonstration Services webpage.













![DSC_0198 [Dan] saves hard drive magnets](https://i0.wp.com/hackaday.com/wp-content/uploads/2015/08/dsc_0198.jpg?w=262&h=174&ssl=1)






















I’m not sure I am fully qualified for such a job. But if such an opportunity came my way I would work hard to find a way to make it work.
That mousetrap fission video was awesome. Need to see it on a larger scale. Maybe with paintballs?
Who here does not have some hard drive magnets laying around?
Ah, the real question is what do you have more of: dead harddrives or salvaged magnets?
The real question is what can you *find* more of: dead harddrives or salvaged magnets?
Same here.
A bit of info on the Chain of Life: https://www.flickr.com/photos/dullhunk/323173954
I believe the lie is that all molecules have tetrahedral structure in the sculpture, yet oxygen (blue) is a bent structure, and nitrogen (green) is trigonal planar. The bond angles are slightly off, by less than 10°, a white lie.
Wow that first picture is the biggest nerdometer I’ve ever seen!
That shop reminds me of some of the better places I’ve worked, though none were quite that nice.
I wish that there were mice in that box.
“mousetrap fission” thank god for grad students!
I had the pleasure of working with Daniel for a few years as his assistant. His job is every bit as cool as you think it might be. My time spent learning from him definitely was of benefit when I became a science teacher.
Harvard’s Collection of Historical Scientific Instruments is pretty badass too. I mean Benjamin Franklin was given the task to rebuild the schools teaching equipment, it has some street cred.
I’d suspect this job is more political than anything. Working at Harvard would be nice because it’s the oldest college in the US.
I really don’t understand why ethanolamine would be called the chain of life. Did they meant to show an amino acid? Those are building blocks of a candidate “Chain of Life” (peptides), but that should be NH2-CHR-COOH (I could argue that nucleic acids are also a chain of life, or even lipids or complex carbohydrates but their structures are very different from this)
(The error may be clearer to non-chemists if look at the simplest amino acid, glycine, whose R group is -H, so it would be NH2-CH2-C(=O)-OH, where (=O) symbolizes a double bonded oxygen jutting off at a different angle from the hydroxyl -OH. In other words: the C on the right should have a double-bonded Oxygen, instead of two single bonded Hydrogens.)
We usually consider -COOH aka -C(=O)OH to be a single substituent group called a carboxyl. The carboxyl end of an amino acid is what makes Amino Acids “acid” (though sometimes the -R group is even more acid). The slightly alkaline -NH2 (amine) group is why we call it an AMINO acid, and the -R group determines exactly which of the many amino acids it is. A few common amino acids deviate slightly from the essential NH2-CHR-COOH backbone, but its enough of a rule to explain how Amino Acids link in chains that we call peptides (aka proteins) — which are a fundamental chain of (known) life.
I wonder whether the “chain is a lie” indicates that the molecule is not a straight “chain” as drawn but forms a circle: The slightly positive hydrogen bonded to the oxygen may “hydrogen-bond” to the lone-pair (pair of unbonded electrons) on the nitrogen giving a circular structure, rather than a “chain”.
Just a thought.
Ugi
Hi! How can we find more jobs like this? My husband applied for this job at Harvard, but too late. The job would suit him perfectly and he is quite disappointed that it didn’t work out. Are there other opportunities at Harvard or elsewhere, does anyone know?
: )