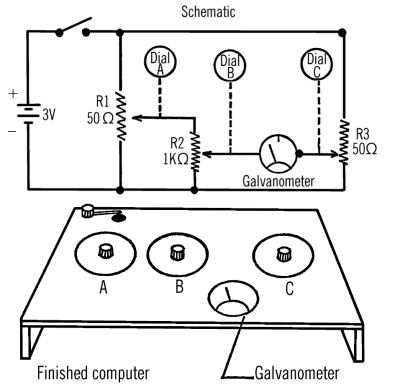
Like a lot of engineers, I spent a lot of time in libraries when I was a kid. There were certain books you’d check out over and over again. One of those was [Raymond Barrett’s] Build-It-Yourself Science Laboratory. That book really captured my imagination with plans for things as simple as a funnel to as complex as an arc furnace (I actually built that one; see diagram above), a cloud chamber, and an analog computer (see below). That book was from 1963 and that did present a few unique challenges when I read it in the 1970’s. It presents even more difficulty if you try to reproduce some of the projects in it today.
The world of 1963 was not as safe as our world today. Kids rode bicycles with no protective gear. Dentists gave kids mercury to play with. You could eat a little paint or have asbestos in your ceiling, and no one really worried about it.
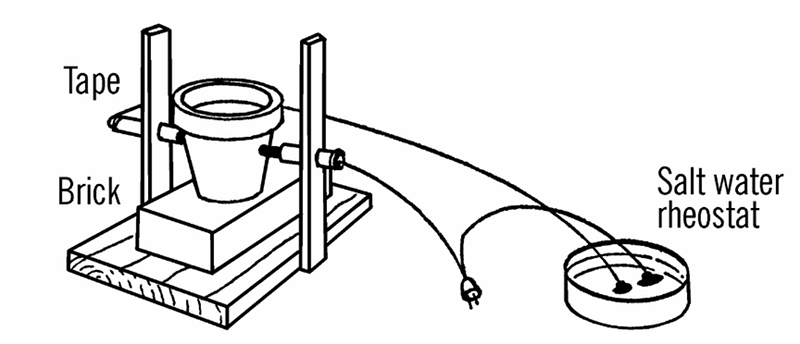
That means some of the gear and experiments Barrett covers are difficult to recreate today or are just plain dangerous. For example, he suggests getting sulphuric acid at the drugstore. I don’t suggest you call your local Walgreens and ask them for it. The arc furnace — which could melt a nail, as I found out first hand — used a salt water rheostat which was basically an AC power cord with one conductor cut and passed through and open glass jar containing salt water! Fishing sinkers kept the wire from moving about (you hoped) and I suppose the chlorine gas probably emitted didn’t do me any permanent harm.
I was delighted to see that [Windell Oskay] has revised and rebuilt this great old book into a new edition. As much of the original as possible is still present, but with notes about how to work around material you can’t get any more or notes about safety.
The book still doesn’t pull any punches, though. On the section for the salt water rheostat, the author notes that there is value in building the device to learn about it, but if you want to use it for projects like the arc furnace, you should use an isolated variac. In fact, he suggests you really ought to use isolation when building the rheostat, too. He even tells you about specific eye protection I should have had with the arc furnace (unfortunately, some 40 plus years too late; fortunately, I got lucky and didn’t have any serious problems).
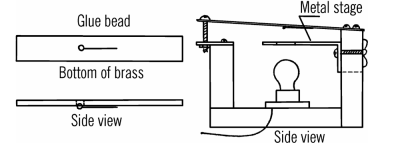 There’s tons of interesting projects and techniques in the book. Need to drill glass? Use a file and turpentine. Want to build a vacuum pump or a vacuum pressure gauge? ([Oskay] cautions about using mercury for the latter.) Want to build a microscope like Leeuwenhoek used? While it would be satisfying to get an old copy of the original, you’d spend a lot of time researching modern sources and replacements.
There’s tons of interesting projects and techniques in the book. Need to drill glass? Use a file and turpentine. Want to build a vacuum pump or a vacuum pressure gauge? ([Oskay] cautions about using mercury for the latter.) Want to build a microscope like Leeuwenhoek used? While it would be satisfying to get an old copy of the original, you’d spend a lot of time researching modern sources and replacements.
Although the book is aimed at kids, and possibly school use, it’s still fun for adults and most modern schools would ban a lot of the more interesting items in it anyway. You can always say you are buying it for your children. Or you can claim you are a prepper and you want to know how to build your own lab after the collapse. Either way, we won’t tell.















I too survived the 1970s, To paraphrase Neal Stephenson’s Cryptonomicon. “Everyone and everything that wasn’t a stupendous badass is dead.”
This is an epic book! Even if you know far more than you should about some of the riskier experiments (:D) there are all sorts of nifty construction techniques and many new tricks to keep a hacker amused. Highly recommend!
I’m old enough to remember when chemistry sets weren’t insipid, and you could get all sorts of useful chemicals at the drugstore.
When I was in grade 4, I checked out “A Boy’s Book of Explosives” (targeted at 12-year old boys). I had nitroglycerine, fulminates, and more. And it suggested buying silver nitrate and such at the drug store. These days bringing a book like that to school may get you a friendly visit from the police, and you may even get to try on their shiny “linked bracelets”.
“I had” -> “It had”. Good books back then.
These days, it’s usually called “The black book of…”. Nowadays, they aren’t targeted at children, but at adults who do not have deep insight into chemistry. They are made with really good instructions on what NOT to do. So, they teach you how to make fulminates, nitroglycerine, TNT, peroxoacetone etc. in such a way that the only bodypart you may lose is eyebrows. (Friend of mine actually wouldnť have lost sight on one of his eyes, has he read the book)
Silly little difference, “I” vs “it”… that little “t” can mean the difference between “oh my” and “jail time”.
Yeah, like when you’re saying you had sex all weekend with a 21 year old girl and your finger drifts to the left of the 2.
A great read for hackers is “Hawkins Electrical Guide”. It’s a 10 volume set; mine is from 1917 but you can still find them here and there. These books cover everything imaginable (at the time) and many things that have been forgotten.
I think you should be able to find all volumes here but I haven’t had the time to check yet,
https://archive.org/search.php?query=Hawkins%20Electrical%20Guide
here you go,
1. https://archive.org/details/hawkinselectrica01hawk
2. https://archive.org/details/hawkinselectrica14hawk
3. https://archive.org/details/hawkinselectrica03hawk
4. https://archive.org/details/hawkinselectrica04hawk
5. https://archive.org/details/hawkinselectrica05hawk
6. https://archive.org/details/hawkinselectrica06hawk
7. https://archive.org/details/hawkinselectrica07hawk
8. https://archive.org/details/hawkinselectrica08hawkuoft
9. https://archive.org/details/hawkinselectric01unkngoog
10. https://archive.org/details/bub_gb_obAVAAAAYAAJ
Thanks for listing them all! Looks like a fine read.
I found a great old book in a pub. From about 1920, a guide to everything electrical back then, mercury rectifiers, electromechanical things, all sorts of stuff. The pub had presumably bought the books by the metre, they had hundreds of random books, just used for decoration. I figured they wouldn’t miss a couple of centimetres worth of decoration.
Some things have not changed.
According to the EPA “the only safe place for Mercury is in a person’s mouth”. Once removed it becomes hazardous waste. You can’t have your extracted tooth if it has a filling, it’s hazmat! This will persist because to admit danger will be the biggest lawsuit in global history reaching back well more than a century!
Reason being that the mercury doesn’t dissolve or evaporate significantly in mixture with the other metals in the conditions of your mouth, which are electrochemically stable.
When the amalgam is handled, such as drilling the filling out, little pieces and dust of mercury get everywhere and when you throw it away it encounters different chemicals conditions where the amalgam is no longer stable and starts to separate.
If they just gave the tooth to you, it would end up in a landfill.
Furthermore, elemental mercury isn’t actually all that dangerous because it isn’t readily bioavailable. Methyl- and ethylmercury are the problematic varieties, and you get many times more of that by eating fish once a week than the levels from dental fillings. Elemental mercury is excreted while (m)ethylmercury accumulate in tissue.
“Ingested methylmercury is readily and completely absorbed by the gastrointestinal tract. It is mostly found complexed with free cysteine and with proteins and peptides containing that amino acid. The methylmercuric-cysteinyl complex is recognized by amino acid transporting proteins in the body as methionine, another essential amino acid.[18] Because of this mimicry, it is transported freely throughout the body including across the blood–brain barrier and across the placenta, where it is absorbed by the developing fetus. Also for this reason as well as its strong binding to proteins, methylmercury is not readily eliminated. Methylmercury has a half-life in human blood of about 50 days.[19]”
https://www.youtube.com/watch?v=9ylnQ-T7oiA&feature=youtu.be
Dax, I believe you are mistaken but maybe this will change your mind.
Yeah, they’re heating the tooth with a lighter. It gives off “smoke”. Big surprise there. You’re buying into well-intentioned but ultimately misleading propaganda.
Protip: that viewing technique is not showing mercury fuming out of the tooth, but hot air rising from it. It’s called a Schlieren (https://en.wikipedia.org/wiki/Schlieren) and the shadow trail on the screen is caused by the change in refractive index as the fluid – in this case air – is flowing by rising up from the heated tooth.
https://www.youtube.com/watch?v=QpP4qM8wcNM
In this video you see the same effect just from a person rubbing their hand to heat it up.
You don’t necessarily need the mirror setup – it’s just more sensitive to smaller differences in the refractive index. Simple sunlight or other bright point source projected through a hole in a curtain will throw a beam of light on the wall, and air currents around objects become visible in their shadow.
If the mercury was actually evaporating at that rate, the amalgam would fall apart within days.
The fact that the “mercury” vapor goes up in a neat trail instead of down – mercury vapors are heavier than air – shows that it’s nothing but hot air and the video is made up.
If fact the video has been debunked elsewhere:
http://quackfiles.blogspot.fi/2005/04/smoking-teeth-truth-gets-smoked-out.html
” the tooth is warmed to body temperature (37 degrees Celsius) in a water bath and the “mercury vapor” coming off of it is made visible by holding the tooth in front of a fluorescent screen and illuminating it with ultraviolet light. The ultraviolet light, strongly absorbed by the “mercury vapor”, shows the shadow of a vapor plume rising from the tooth.”
“water vapor ALSO strongly absorbs UV light. However, this does not rule out mercury vapor.”
” if what we saw was actually mercury vapor coming off those teeth, and not just water vapor, it should have been SINKING rather than rising – even at 37 degrees C. Therefore, the video DOES NOT show mercury vapor rising off the tooth, only water vapor.”
To be honest, the smoking tooth video does not look even remotely like what you could get with the schlieren technique. Meanwhile, the colour of the fluorescent screen is pretty much identical to the colour of the luminophore used in the thin-layer chromatography plates I used to handle, which has a peak of absorbance around 254 nm — exactly what you would need to visualise mercury vapour, among other things.
As for the “debunking”, the author doesn’t know what he’s talking about. The density of saturated Hg vapour at room temperature is like 0.02 g/m³, about 50 times less than the density of air. So even if the fumes coming from the filling were saturated (they certainly aren’t), they wouldn’t add appreciably to the density of air, which would therefore move up.
I had an old one, something like Readers Digest Junior Treasury, and between insipid stories and lame puzzles it had stuff like how to build a carbon microphone, and mini rockets from match heads and things, think it had some electrochemistry and crystal growing with currently VERBOTEN chemicals but can’t remember exactly.
I remember blowing out every fuse in the house by wiring two large carbon electrodes–from carbon-zinc cells–straight across the mains; trying to build a carbon-arc lamp, of course.
Ah, those were the days. How else, except from a potentially deadly experiment, to learn a lasting lesson about ballasts being a requirement for negative-resistance circuits? (Hey, the house DID have fuses)
“Those the gods hate, they keep alive.”–anon
I remember poking nails into things like hotdogs, potatoes, and other random objects…and then wiring them to a dryer plug. We used to run around to the electric meter and see how fast we could make it spin.
Also tried to build a pulsejet in high school…parents shut that down after a “small” explosion.
These days cops and CPS would arrive and wreck things up…it’s a pitty.
Heh heh, I had a bit of a fascination with pulse jets too, experimenting with tin can contraptions, I never managed to get one pulsing more than two or three times… though there was that one that shot off across the room and nearly set fire to the curtains…
I do remember discovering this book at the library. I think I contributed significantly to filling the checkout card inside the front cover. I got into a significant amount of trouble with all the experiments in electricity and chemistry. What really got me inspired were the Amatuer Scientist articles in the back issues of Scientific American by C.L. Stong. The illustrations of the apparatus were amazing!
The Amateur Scientist collection is available on CD:
http://www.surplusshed.com/pages/item/m2071.html
Also, “700 Science Experiments for Everyone” is readily available, quite inexpensively. The old 1958 version is the dangerous (and good!) one…
A variety of books available here http://www.librum.us/index.htm
That is an amazing collection there, but are you sure we muggles are allowed to read those books?
;-)
The book I had was “Exploring Science in your Home Laboratory” by Richard Harbeck. It too was from 1963, but this was a Scholastic Book. I somehow ended up with a used copy later in be decade. Shows you how to make a workbench and various things you might need fir chemistry, biology and physics. More fun than a Carolina Scientific catalog, since you could make things yourself.
I seem to recall a Golden Book that went into making lab equipment, but I don’t know where that might be if it existed.
Of course, once the switch to hobby electronics, there was a whole lot more one could build.
Michael
https://archive.org/details/Golden_Book_of_Chemistry_Experiments_How_to_Set_Up_a_Home_Laboratory_Robert_Bre
Also,
https://archive.org/details/LaboratoryInstruments
https://archive.org/details/agriculturallabo00waterich
https://archive.org/details/laboratorystudyo00jone