Micro-what? Microfluidics! It’s the field of dealing with tiny, tiny bits of fluids, and there are some very interesting applications in engineering, biology, and chemistry. [Martin Fischlechner], [Jonathan West], and [Klaus-Peter Zauner] are academic scientists who were working on microfluidics and made their own apparatus, initially because money was tight. Now they’ve stuck to the DIY approach because they can get custom machinery that simply doesn’t exist.
In addition to their collaboration, and to spread the ideas to other labs, they formed DropletKitchen to help advance the state of the art. And you, budding DIY biohacker, can reap the rewards.
In particular, the group is focused on droplet microfluidics. Keeping a biological or chemical reaction confined to its own tiny droplet is like running it inside its own test-tube, but because of the high rate at which the droplets can be pumped out, literally millions of these test-tubes are available. Want to grow hundreds of thousands of single cells, each in their own environment? Done.
The DropletKitchen kit includes an accurate pump system, along with high-speed camera and flash setups to verify that everything’s working as it should. Everything is open-source, and a lot of it is 3D-printable and written in OpenSCAD so that it’s even easy to modify to fit your exact needs. You just need to bring the science.
This is a professional-grade open source project, and we’re excited to see it when academics take a turn toward the open. Bringing cutting edge processing technologies within reach of the biohacker community is a huge multiplier. We can’t wait to see what comes out of this.

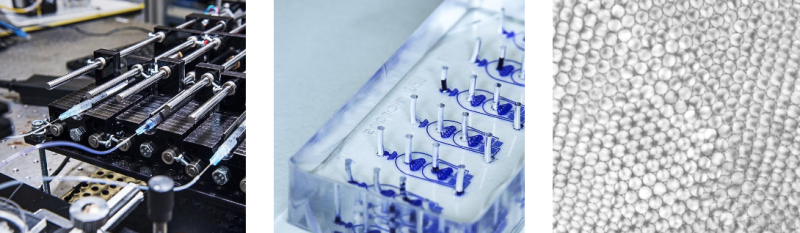














Hmmm potential to 3D print biology from this??? Down the road, not next Tuesday.
Last week, really. It might be a while before the killer application (so to speak) is worked out, however.
Triffids to patrol the border obviously.
There probably needs to be a new name for “killer application” when it comes to bio-technology
microfluidics has been diy tier for anyone with access to a laser cutter for years. read more journals.
But no one even claimed it was new? O_o
It’s just a report on one group and how they transitioned from bought to DIY. The main news is the community they created behind it and the implementations they came up with.
yeah but there’s nothing even new here. This is how every pub looks. Everyone builds a crappy inverted microscope to watch liquids through a CCD camera somehow. I think it’s only notable because HaD people are not microfluidics people so the existence of these kinds of experiments is news to them.
The only big difference I see between this and every paper from 1990-2010 is the light strobing. Usually you try to just buy a higher speed camera if the fluid velocity across the field of view is high. These cameras are expensive, this may provide some good benefits for some setups.
I also would recommend you just buy your own syringe pump and not make one. You would not try to make your own transistor, for instance. Used harvard apparatus pumps are very cheap, and for droplet gen I think constant pressure at the fluidic junction is more important than constant volume rate anyway, but I could be wrong there.
“You would not try to make your own transistor, for instance.”
Been there, done that. (without much success, diodes worked, though)
We’re on Hackaday, and not on “How to buy used, very cheap harvard apparatus pumps”.
As an owner of a laser cutter and interested person, please provide a journal. I’ve been failing at microfluidics for years now.
http://www.nature.com/nprot/journal/v10/n6/full/nprot.2015.051.html
http://scitation.aip.org/content/aip/journal/bmf/4/3/10.1063/1.3487796
http://pubs.rsc.org/is/content/articlelanding/2002/lc/b206409j
Personally I find it easier to use templating with PDMS.
PDMS for the win. Just do the litho on a wafer (find your local nanofab at your favorite research institution) and expose KMPR or SU-8 resin onto a wafer to make a mold. Pour PDMS over the top, cut it off after it sets, and use O2 plasma to bond it to your favorite glass slide. Usually it’s 125mm or 150mm wafers, you get as many designs as you can cram on the wafer.
It does flex though, so if you need high structural rigidity, there are other more expensive things you can do…
on another note, there are places that do reel-to-reel microfluidics too if you can eat +/-5um height tolerance too. This way you don’t have to have access to all the semiconductor litho tooling, plasma cleaner, etc.
“Bringing cutting edge processing technologies within reach of the biohacker community is a huge multiplier. ” Grey goo? This is how you get grey goo.
Don’t worry, we’re years off grey goo yet. This is merely how you get green slime.
“Keeping a biological or chemical reaction confined to its own tiny droplet is like running it inside its own test-tube, but because of the high rate at which the droplets can be pumped out, literally millions of these test-tubes are available. Want to grow hundreds of thousands of single cells, each in their own environment? Done.”
The only problem I see is containment. Acoustic suspension perhaps.
Too bad the github repo doesn’t really have anything useful for someone wanting to start out… they don’t even talk about microfluidic fabrication… IMO clickbait title for engineering nerds :/
Entirely copied from previous work back in 2010 or so, nothing new here, as previously stated. Looks to me if the authors have simply taken ideas from elsewhere. Disappointing, to be honest.