If you came here from an internet search because your battery just blew up and you don’t know how to put out the fire, then use a regular fire extinguisher if it’s plugged in to an outlet, or a fire extinguisher or water if it is not plugged in. Get out if there is a lot of smoke. For everyone else, keep reading.
I recently developed a product that used three 18650 cells. This battery pack had its own overvoltage, undervoltage, and overcurrent protection circuitry. On top of that my design incorporated a PTC fuse, and on top of that I had a current sensing circuit monitored by the microcontroller that controlled the board. When it comes to Li-Ion batteries, you don’t want to mess around. They pack a lot of energy, and if something goes wrong, they can experience thermal runaway, which is another word for blowing up and spreading fire and toxic gasses all over. So how do you take care of them, and what do you do when things go poorly?
Gravity of the Situation
This video happened 2019/10/20 in Shantou, China, and shows a charging electric scooter experiencing some rapid unscheduled disassembly. Play with sound, but maybe turned down a bit. Notice also the fire extinguisher in the bottom right, as if this was perhaps not an unexpected event.
What’s happening is thermal runaway, where the battery gets too hot and so it starts self-destructing, igniting the electrolyte, which then releases energy, creating more heat, which causes more self-destruction of nearby cells. This can happen in a variety of ways:
- Short circuit: Either through damage from an impact or piercing as with a knife, if the layers lose their separation, a short circuit is created, allowing a lot of energy to move very quickly, and generating a lot of heat in the process. It’s also possible for batteries over time to develop dendrites, sharp crystals similar to stalactites in caverns, that can pierce a separation layer to create an internal short.
- Overcharging: When the battery is charged above its maximum voltage, it can generate heat.
- Excessive current: during charging or discharging.
All three are from lots of energy moving very quickly and generating too much heat. If you’re interested in exactly what is happening inside the lithium cells, we’ve covered this in more scientific detail in the past. But even without understanding the chemistry we’ve seen the consequences through notable events like when the failing Note 7 batteries were causing problems a few years ago and led to changes in airline policies with certain devices.
In addition to the threat of fire, toxic gasses are released during this fulmination. Cells contain a quantity of fluorine, which reacts to form hydrogen fluoride in significant quantities, making the smoke from an event an immediate danger to life or health, especially in confined spaces.
Safety Precautions: Venting, Cell Protection, and Monitoring
Electric vehicles have been rolling off assembly lines and into garages at an ever-increasing pace. The energy potential in each vehicle is immense and there is much research into Lithium Ion battery chemistry aimed at significantly improving lifetime, and the integrity of the cells. There has also been a lot of research into what levels to charge and store the battery. It shouldn’t be charged up to 100% all the time, with a 50-70% charge recommended for long storage periods.
Knowing HOW the batteries detonate, we can do some things to discourage them, and design that into the cells and packs. At the cell level, the first protection is in the separator itself, which keeps the anode and the cathode from touching each other. This insulating layer is designed to be porous enough to absorb the electrolyte and allow the lithium ions to pass through, and when heat builds up can even close the pores to shutdown transfer. But its main job is to keep the peace between the anode and cathode.
Outside of the chemistry, the cell is usually covered in some protective layers, which can be a metal pouch for aptly named pouch cells or a metal canister for cylindrical cells. Both try to avoid punctures, and contain any gases that may develop. They may need to vent that gas if too much pressure builds up, so enclosures should be able to expand a little to accommodate a swelling battery, and provide a means for gas to vent safely if it does build up enough pressure. If the gas doesn’t vent then it could explode. It’s coming out either way.
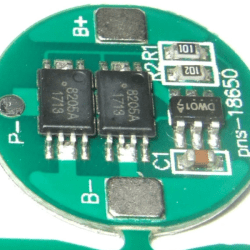
Another safety precaution is the protection circuit, which can be applied to individual cells for the most safety, or to multiple cells if they are balanced. We covered a DIY cell protector a couple years ago. These circuits can monitor temperature, current, and voltage, and shut down the cell if any of them get outside of safe ranges. Most battery packs will have these circuits built into them by default; you’ll have to specify a bare unprotected pack if you really want one.
Outside of the pack itself, it’s generally wise to have additional protection circuitry that can prevent the pack from ever getting to a point where it needs to shut itself off. If the device can sense the problem before the pack shuts off power, it can correct it and still provide an interface to the user and shut down gracefully. Monitoring current, temperature, and voltage on the main board can help with this.
Treat Them with Care and Know How to Dispose of Them
Naturally, they should not be used or stored in such a way that they will be exposed to high heat. They should be protected from impact, especially sharp conductive impacts. They should be charged carefully and under supervision with a reliable charger. For larger packs, they probably shouldn’t even be inside a residence when charging, in case of occasions exactly like the video above. Pouches that are puffed are no longer safe and should be disposed of. Batteries that are old and no longer work can still be dangerous.
Here’s what can happen when a pouch battery gets cut or punctured. This is an extreme example; but even nicking it with a knife can damage a separator layer, which may eventually allow the anode and cathode to touch and cause an internal short.
Disposing of Li-Ion batteries isn’t difficult, but most people aren’t doing what they should be. The battery should be separated from the rest of the gadget in as safe a way possible. (Chuck the power tool but not the battery pack, but don’t break your phone in half trying to extract the battery.) Then the leads should be taped so that they can’t touch each other or short on some other conductor. Bring them to a place that can recycle them, like many home improvement stores or electronics recyclers. Throwing them in the garbage can be really bad because of the possibility that trash compactors and other processes will damage them and cause garbage fires. Plus we want to avoid all of the nasty chemicals ending up in the landfill.
Emergency Procedures with Lithium-Ion Batteries
There’s some conflicting thought here. On the one hand, your safety and the safety of others is paramount, and the gasses released by a burning pack are dangerous and possibly toxic, especially in confined quarters. If things are bad, you should get out and alert others and the authorities. On the other hand, you don’t want to lose everything if you have a fighting chance, quick action could resolve the situation, and you’re able to make your own decisions, or maybe you’re on a plane and you can’t really escape.
 Lithium-ion battery fires can be class A, B, or C fires. They will burn on their own, are made of combustible materials, and have flammable liquids and solvents. When they involve energized electrical equipment (the charger), they attain class C. With things plugged into a wall, you do not want to use water, as that only makes electrical fires more dangerous. However, for packs alone and not energized externally, water is an effective solution. It puts out the fire, and it reduces the temperature of the pack so that it can’t re-ignite itself. Any fire extinguisher should work to kill the fire, but as long as the temperature stays above the flash point of the electrolyte, it can restart itself, possibly hours, and in the case of electric vehicles, days later.
Lithium-ion battery fires can be class A, B, or C fires. They will burn on their own, are made of combustible materials, and have flammable liquids and solvents. When they involve energized electrical equipment (the charger), they attain class C. With things plugged into a wall, you do not want to use water, as that only makes electrical fires more dangerous. However, for packs alone and not energized externally, water is an effective solution. It puts out the fire, and it reduces the temperature of the pack so that it can’t re-ignite itself. Any fire extinguisher should work to kill the fire, but as long as the temperature stays above the flash point of the electrolyte, it can restart itself, possibly hours, and in the case of electric vehicles, days later.
You may be wondering why it’s not a class D fire, which is a combustible metal fire. If we were talking about primary lithium batteries, the non-rechargeable kind, then it would be a class D fire requiring a dry powder extinguisher (or smothering in sand or other non-combustible material that isn’t water). But the amount of Lithium in a Lithium-ion battery really isn’t high enough or concentrated enough, and it just never gets to a point where the lithium itself is igniting.
In a cases where you’re concerned a battery might be in a dangerous state, the best way to handle it may be to first dump it into a container and take the container outside to a safe place where you can then work on putting out any fire that develops without endangering yourself or any buildings.
The video of the electric bike fire mentioned earlier shows a surprisingly good reaction. The person unplugs the pack first, giving them more options and reducing the likelihood of exacerbation of the conflagration. You can see them grabbing the fire extinguisher on their retreat, probably so they can arm it in safety. Then they return and put out the fire. It reignites and they hit it again. Someone opens the door to get some ventilation and remove the smoke.
In the aftermath they should bring the bike and themselves outside where there are no combustibles and the pack can exhaust whatever fumes it has remaining. They should air out the entrance area without spending much time in it. And they should soak the battery for a long time in water to cool it down.
A much more detailed guide can be found in this paper on Li-Ion Battery Fire Hazards and Safety Strategies. As we continue to surround ourselves with Lithium battery powered devices, having a working understanding of the treatment, the warning signs, and the appropriate response is a good idea for everyone.















That is a clever and very thoughtful first paragraph. Just wanted to say that.
I keep a couple of aerosol can type Halon fire extinguishers on top of the cans I keep my lithium batteries in for storage. The idea is if I’m not home and a fire did happen the plastic top will melt and the extinguishers will self deploy, maybe wishful thinking but better than nothing at all. Most grocery stores sell those plastic top extinguishers not Halon I just happen to have a few cans of Halon ones! Fireproof battery bags are also a good thing to have and of course having a fresh fire extinguisher next to where you charge is best practice and always babysit them especially High C lipos.
https://www.bat-safe.com/
“©2023 by BAT SAFE LLC”
They sell time-travel too?
Respect to you for reading the entire page.
It is a division of Wayne Enterprises, after all. What did you expect?
“The video of the electric bike fire mentioned earlier shows a surprisingly good reaction.” — Why surprising? While electric scooters (both their benefits and their hazards) are still sort of new in the west they’re very common in China and have been for a numbernof years. I expect that the knowledge of how to put out such a fire is fairly widespread. (I expect articles like this have long ago circulated in Chinese).
cars have been common in the “West” for over a century now, yet most drivers lack even the basics on how to keep one from burning down to a crisp once the flames show up…
How often a car catches fire vs. how often a Lithium-powered electric doodad does: details DO matter mate.
I know one should not use ones own experience, but I have seen more car burn then batteries, just saying.
That said, I don’t try to downplay they danger with batteries.
Once one of these light, you’re pretty much a spectator until it runs out of fuel, much like a magnesium fire. You don’t have enough water, and even a 100lb extinguisher isn’t enough.
It seems indeed better, that if the thing ignites to give it a minute or so to expend it’s energy.
Seems much better then emptying your fire extinguisher.
I also like MikeR’s advice. of putting batteries & charger in a fireproof box together with an extinguisher (plastic bottle with water).
A few years back there was an article on Hackaday about fire risks of 3D printers, and ways to (automatically) extinguish them. May get some inspiration there…
burning lithium and water in a confined space…what could possibly go wrong?
If you read the article, lithium is not actually burning here
Water and lithium produces lithium hydroxide and hydrogen gas, at high temperature.
it is however the reason the damaged battery becomes basically pyrophoric
Don’t worry, commenters don’t have to read the article to demonstrate their failure to comprehend Lithium battery chemistry.
I live on a boat (41′ Chris Craft Commander 410) and the below deck where the two Mercruiser 340’s live is watched over by a halon flooding system. This will not cool any lipo fire but will purge oxygen well enough that once the stored charge dissipates nothing else should be able to be ignited. That’s my hope anyway :), what with two 150gallon gasoline tanks under the aft stateroom king size bed. I joke with the Mrs about our His and Hers bed buddies.
No, it’s no magnesium fire, it is also no lithium fire. The Li is already in ion form. When you read the article, you know, that the organic solvent electrolyte burns. Ignited by the electrical energy in the battery. The typical scooter has about 300Wh to 500Wh battery capacity. 500Wh will raise the temperature of your “100 pounds” (exactly 42,8kg) of water by 10°C.
The electrolyte soaks the separators, that will probably not be more than 2-3cm³. Even if it would have the energy density of pure gasoline that water would be about another 20°C more – far from boiling.
Cool it, dilute it and keep it from re ignition by cooling.
In the emergency you can use even a water or foam extinguisher on the connected (charger) vehicle, if you keep at least 1m of distance. You will not get a shock and there is only some chance to trip the breaker or GFCI. Of course, if possible cut the power.
A CO2 extinguisher is not that good here as it has much less cooling power than water. It can not prevent the re ignition.
The best comment so far. Thank you. There are no start one can click on, I would give you five.
So thanks for some sanity here among all manguessing people.
Battery safety standards, directives, and regulations are a mess. Some end-use standards and directive cite IEC 60086-4, but this is for primary batteries. Other standards and directives cite IEC62133, but this is not a harmonized standard, and can be considered to be an incomplete test standard.
UN38.3 and IEC62281 are decent standards, but are for transport and not for functional testing.
UL1642 is a decent test standard, but industry is attempting deprecation. One of the many issues with Li battery test and certification is the poor implementation of test plans and poor reports. UL1642 is a decent standard with reasonable and representative tests. But then there are (IMO poor) test reports such as this:
http://www.globtek.info/certs/2GL-523450-G2107(R)/NEMKO-REPORT-2GL-523450-G2107(R)%20UL%201642%204th%20Edition%20CELL%20ONLY.pdf?redir=no
Electrical equipment engineers should not only test and design per the scoped end-use standards, they should reference the component standards in the end-use standard’s appendices. (I am looking at you IOT designers,as I have yet to review a compliant and safe battery-powered IOT device).
Finally, “Lithium-ion battery fires can be class A, B, or C fires” should be “Lithium-ion battery fires can be class A, B, *AND* C fires”. For almost ten years, I have caused or witnessed at least one Li battery-caused fire per month. The author’s advice for handling these battery fires is good, but not complete
Thanks for your addition. I considered my words carefully with the boolean operator. And implies that any fire is all three, whereas or means it could be any one or combination.
For me personally, at least, I find that plain English “or” is ambiguous but frequently reads as “xor” without a bunch of extra disambiguating baggage. I tend to use “and/or” to mean “Boolean or”, But I’ll grant I’ve done no survey of if this is actually necessary or effective, so. Take that as you will, I guess.
That is to say literal “and/or” (and-slash-or), speaking of ambiguity…
This is a very timely article for me. I just bought a battery charging circuit and battery protection circuit from The Usual Sources (banggood.com) to manage a 12 cell 3s4p lithium ion battery that was recovered from a laptop. It was brand new but its charging circuit wouldn’t communicate with the laptop, so the laptop thought it was at 0% and would shut down despite having a full battery. This time it’s going to power some ham radio equipment and lights on a recumbent trike. I plan on charging it outdoors and inside an ammo can designed for the purpose. If it asplodes, hopefully the ammo can can contain it.
I’d advise you to read about battery balancing: https://en.wikipedia.org/wiki/Battery_balancing
Your pack is probably damaged either in circuitry or cells. Charging them in series will do no good to the pack and it will be a danger to deploy indoors after charging.
You can buy cheap balancing gear online if you wish to build/reuse your own packs.
Yup the circuitry is defective. But, the stuff I got should take care of it by replacing it. I’ve read that article as well :)
It doesn’t help that charge management chips are oddly stagnant, aside from the ones only available in BGA/TQFP which don’t seem popular in the cheap crap on eBay.
Nearly ALL common charging chips are set to the wrong charge voltage. They charge to 100%, which is really not ideal for anything I can think of.
4.2v is not the proper charge voltage for a lipo. Manufacturers seem to think it is, but if you ask people if they really want that extra 10% capacity, they’re probably going to prefer slightly less capacity and less degradation of their (Usually nonreplacible!) batteries.
Cell phones, laptops, pretty much everything charges fully, for no real reason.
Doing power management is usually the hardest part of any project I do, because most everything related to it kinda sucks.
My car charges itself to 80% but I wish my phone and laptop had that option too.
That depends. In my (already quite old) Galaxy S5 the battery is charged to 4,35V. But swapping it is a matter of seconds. I already did it once, after about 3,5 years and I am still very happy with this phone. What I do is avoiding to charge it (overnight) when it has more than about 60% left in the evening. That is often the case after one day.
I think Apple is not charging 100%, which is one more argument for you. And yes, I know about too. And storage should be like the article say, 70% or lower.
Hi geocrasher, charging outside sounds like an interesting idea, but for those that are living in apartments, not
so much. As a ham operator myself who will soon be getting a reverse recumbent trike, I am interested in
your setup. Are you considering solar for charging?
The trike will not be electric, but will have electric accessories: lighting mostly, and a repurposed smart phone for a bike computer. Check my site https://miscdotgeek.com for updates :)
For that purpose I think a wheel-hub dynamo (generator) would be the best option. It has nearly no extra mechanical resistance if no power is taken from it.
I’m Going to jump in the jargon section as I dont solder circuits. Do External Hard Drives have the same Fire Risk? Could Internal Sensors Shut Down during Data Transfer? What about Tesla’s ‘New’ lithium iron phosphate battery? Tesla Plans to Use Iron anodes in place of nickel. Isn’t Graphene Already Employed in the Separation Layer of Roll Type Cells? There Seems to be a lot of Beating Around the Bush when it comes to how cells are made in a traditional Roll Type Layout that Sounds like Business as Usual. A ‘Carbon’/Fiberglass Tube Would Have More Insulating Properties than a Metal Case
DIY Li-Ion Batteries is not much different to DIY medication – you might be lucky – or you die
– this is how nature eliminates .
I have been in the Li-Ion battery business for the last about 15 years and hav seen a few fires
– with packs of one cell to 400+.
DIYers will never have the experience to understand it – how can they.
Otherwise they would be professionals.
I can only hope that only the better-knowing hobbyists pay the price – and not their families and children.
This is a bit toooo black and white, isn’t it? Not to question all your years of experience, but with a few very basic counter-measures Li-Ion and Li-Poly seems quite safe:
– always stay well within the batteries specs
– do not use the so often stated 2.4V minimum discharge voltage. I use 2.9/3.0V as the lowest discharge voltage.
– do not charge above 4.2V, better use 4.1V as cut-off
– do not discharge with higher max currents than stated in the datasheet
Anything to add?
Cheers, Jan
How many batteries have you built and used? And stressed?
Overcharged, shorted, discharged them with too high currents, penetrated them with tools, used packs with badly balanced/deep discharged cells…
Like everyone should who handles those packs regularly. to see how they behave.
Making some fuzz and black art out of it doesn’t help anyone for sure. Write down your knowledge and let us learn.
there’s a fair bit of variance between different lithium cells, so I don’t necessarily agree with all of your numbers in many cases, but in general that’s good advice.
In general, a lot of 18650 cells are spec’d with a much lower minimum discharge voltage than a typical lipo pouch cell, and given that you’re discharging them at rates comparable to what they’re tested with, and spec’d in the datasheet, you can go a bit closer to the recommended operating spec without danger. Keep in mind the difference between “Absolute maximum/minimum” and “Recommended” specs. Also, deratings with temperature, etc (often overlooked). With a lot of those cells, if you choose a cutoff which would be appropriate for a cheap RC pouch pack, you’re calling it empty with 1/3 the capacity remaining.
OTOH, when I’m dealing with fairly high currents and RC packs, I’d actually be a little more conservative than your 2.9/3.0V, especially if you’re calculating from the whole pack voltage. Once you’re down to 3.6V or so volts/cell under light/no-load conditions, you may as well stop, because they usually drop like a rock below that point and you’re likely to over-discharge a cell, since RC stuff is invariably top balanced, and not necessarily super consistent cell to cell. I would rather lose 30 seconds per flight on my quadcopter, than be continuously abusing the weakest cell.
This actually brings me to an often neglected point. Don’t assume that your cells are equal capacity, or that they’re at equal voltage at any point other than when they’ve been freshly charged and balanced.
It’s far too common to use pack voltage to decide when a pack is “empty” and this is often calculated as (num_cells*cell_cutoff_voltage) . Unfortunately, with many cells the discharge curves get pretty steep towards the end, so you can have a perfectly top balanced pack show very different voltages near empty.
As an example, assuming 3.3V/cell as a safe LVC for a Lipo pack means 9.9V would be your pack cutoff voltage, but it’s not super unusual for one cell to hit empty first, and due to the steepness at the end of the curve, you might end up with 3.58, 3.53, 2.79 as the weakest cell falls off the cliff shortly before the other ones follow.
You need to either use a per-cell monitoring/LVC which shuts off the pack as soon as any one cell passes cutoff, or you need to use a more conservative number.
A less used alternative, which may actually be better would be to bottom balance. Does anyone know why this isn’t more popular? It makes more sense in my head, but I don’t really see it done. I suppose it’s probably just to allow dumb generic chargers to be compatible with generic shunting BMSs
Thanks a lot for your very helpful response!
You’re totally right with everything you mentioned, my post was meant as a general starting point to not accidentally set your house on fire :)
@NickW I will very likely put your info into my latest project, the 34V (8S) LVD circuit. There I have exactly that problem, especially when using older packs which are not well balanced anymore.
A per-cell attempt would still be the best option to be sure, it’s just very tricky to embed this without very specialised chips which are super expensive…
do not bite into iphone batteries – see video from China This video happened 2019/10/20 in Shantou, China, and shows a charging electric scooter experiencing some rapid unscheduled disassembly. And do not sit on one (same video series)
How can they? Research and learning, same as with any other subject?
Like anything that involves a lot of energy, batteries need to be respected, but your implication that a hobbyist can’t have this knowledge (and experience) is pretty condescending.
There are certainly DIYers who are under-prepared and under-experienced, and who shouldn’t be messing with lithium batteries, but coming on hackaday and just saying “Go home and leave it to the professionals” is kind of antithetical to the purpose of this site.
A warning about some of the dangers, and the differences between lithium ion, and other batteries that DIYers may have experience with is certainly warranted, but I don’t think this is exclusive to the DIY community either.
I’ve seen a lot of “professional” designs that make huge mistakes, and honestly I’m surprised that fires aren’t far more common in cheap consumer electronics.
Yes I know about a 2p4s tool pack – from a really expensive professional press for press-fit water pipe connections – that hat no BMS or balancing at all. So the only possibility for UV cutoff was pack voltage. Obviously a design copied from the days of NiCd.
As a result one cell pair hat 0V and the others were weak, one cell of one pair having zero capacitance left. I wanted to reuse some cells for a light duty intermittent load (2s or 2s2p). But after measuring them decided to discard them, none of them was over 1Ah. Rated capacity was about 1,5Ah.
And that’s why I neither build my own Li-ion cells from graphite powder and lithium salts nor synthesize my own acetaminophen. I’m perfectly fine buying those from competent manufacturers and using them according to well-known safety practices, though.
It is, in fact, possible to understand something without having spent 15 years doing it as a job, through the obscure and exotic practices called “education” and “research.”
DIYers build mains-powered circuitry without electrocuting themselves (usually), build boats and sail them without drowning (usually), build planes and fly them without crashing (usually), even build fusion reactors without giving themselves cancer (usually). We also cross the street without getting run over by a bus (usually). Hazards are everywhere, and managing them is part of life. Ignition-happy batteries are a more dramatic hazard than some others, but not some kind of apocalyptic horror that may only be touched by the anointed few Serious Professionals lest the world be consumed in hellfire.
TL;DR: It’s a bloody battery, not a fission reactor with a large positive void coefficient and no containment building. Lighten up, Francis.
> We also cross the street without getting run over by a bus (usually).
Only in Europe. In the US amateurs aren’t allowed to cross the road except at managed crossing points.
However, thanks to careful instructions, most British children attain this skill before reaching high school and are awarded a licence for it (a large badge with a ‘10’ on it, usually awarded around their tenth year).
The art of “not feeding the trolls”, no matter how experienced they may state to be, seems to be lost in time.
He does not want to share his high elf knowledge, even is he had it, thats obvious from the very beginning of his post.
“Otherwise they would be professionals.”
Which is a vague term banded about that means different things to different people.
Lots of people claim to be professionals but are totally inept at what they do.
Business is full of them.
The ranks of the hobbist are also full of people with vast knowlege in their field that either dont want or cannot get employed as professionals for various reasons that have nothing to do with their knowledge of the subject, perhaps they are “not a team player” or dont wish to relocate or have a better income in their daily gig.
Yup yup yup, out of all that call themselves professionals in a field, I have observed about 20% to be truly that, about 60% to muddle through on voodoo rote knowledge, and 20% to be an absolute menace to society.
Is this the hobbyists or the professionals that you are talking about? :P
Should be applicable on both categories, both professionals and amateurs.
Professional is (just) a person that get payed for doing a task, that amateurs do without pay. Usually it also have a certification, which give some lower bond on knowledge. But not 15 years experience and Civil engineering level knowledge in the subject.
And yes, some stuff should not be done by amateurs like, but not exclusively, 50V or more electrical systems with high current. Against the law and insurance company rules. For good reasons.
I think nearly nobody tries to DIY construct lithium ion cells. Connecting them together to packs is no problem. Of course you should know what you do (as with anything in life) and use the necessary protection and or balancing circuitry.
it would be nice to see an article on li-ion vs. lipo and how to use them in projects safely. quick google search is mostly blogspam or has flat out wrong facts
And li-fe too, it seems to be getting popular here and there.
Agree. Or someone can post a link if they know a good source.
I have mounted two chargers on the outside of a steel box with extra fireboard on the inside. The charge cables and (extended) cell sense/balance connectors reach inside the box, so I can charge two batteries with the box closed. Usually all my Li-ion batteries are also stored in the box: https://www.dumpstore.nl/images/4653092_m.jpg
You will note that for all the pyrotechnic effects in both videos that there was not much of a fire outside of the item that was burning. The second video with the axe, the piece of wood the battery was taped to was not even charred.
Not to say something bad could not have happened but it was interesting that in both examples nothing really bad did happen.
Electrical explosions are often fun (when viewed at a safe distance and someone’s paying you to make them happen).
Sadly never managed to blow up a lithium battery. Well, maybe 1 fire involving a badly labelled battery holder causing some meltyness.
But a nice big kaboom can be made with lithium thionyl chloride batteries. Beware them, especially if trying to make a kaboom.
Got away with a ticking off after letting loose a 1/2 AA sized one at a test facility where rather large bangs were a normal thing.
Never put one in the same pocket as your keys. A short circuit is all they take!
We should differentiate lipo batteries from lithio ion batteries. Lipo batteries don`t explode, but litio ion are the ones that explodes instantly. You can see that the lipo battery starts to catch up pretty slow. On the scooter, everything is quite explosive. Each explosion is from each cell I guess.
This difference is important. That is why we have protection circuits on the 18650 and nothing on the LIPO battery. This doesn`t mean that a LIPO battery is not dangerous though, but it explains why we need to be careful with the 18650 cells.
Also, LIPO batteries should be discarded as a e-waste and not thrown into the bin, but if you want to be safe and you know that you can`t go immediately to an electronic garbage collection point, while you don`t want to pile up dangerous stuff, which are also bloated and looking dangerous, you can safely submerge them completely into water with salt and leave them for a while. After that the battery won:t hold any charge so short-circuits are not a problem.
An lithium ion battery, as I said, leave them alone with their hard packaging and protection circuits and discard them safely.
This is mostly bad info.
Lipo batteries definitely can explode, and 18650 cans generally have safety vents so they don’t explode (it’s never a guarantee though). In general, both will normally aggressively burn, rather than actually exploding, but YMMV.
Many 18650s are unprotected, and the reasons for lack of protection on lipo batteries is the same as for those 18650 cells. They’re intended to be used with other circuitry to protect them. (Special chargers, LVC circuits in lipo powered devices)
18650 cells, or 18650 based packs should still be disposed of safely. Ideally they should be recycled if that’s available where you are, and if not, should be taken to an e-waste depot, which should deal with them appropriately.
I’m not sure what you mean by “discard them safely”, but if you’re implying that the packaging and protection circuitry means they can be thrown in the trash, you’re wrong. In all likelihood they’ll end up in a compactor, or crushed by a bulldozer in the landfill and start a fire. I’ve even had a lithium pack catch fire just from water getting into the protection circuitry, so that’s another potential cause.
I charge mine inside 8” CMU’s , hollow blocks . Then a bag of sand on top . Anything goes south , plastic melts and sand will cover everything . Oh yes , bottom of course non combustible material .
Unlike in the movie, these Secondhand Li-Ions can be very dangerous.
B^)
My company recently did a battery fire training day where we went to a controlled, safe site and intentionally started lipo and lion battery fires using a variety of methods.
Results:
Puncture fires of eventually charged batteries tend to be nice and flamey but short lived. Mostly just a lot of smoke.
Over charged or especially charged at incorrect voltages are massively explosive with a lot ofnflame.
Shorts due to crimping cables in box lids, etc are slow to go up since the battery internal resistance is higher than your think.
Observations about putting them out:
Water is absolutely the best option for a variety of reasons:
1: once it goes south, the battery is self destructing. There is no stopping it. Even in a bucket of eater, the battery will continue to consume itself for a long time. Unlike other options, water continues to regulate the temperature, controlling the destruction and dousing fire.
2: when fully submerged, water acts as a filter to some of the worst of the chemicals escaping into the air your are breathing. After, there is a thick film of sludge and foam floating on top which was trapped and collected rather than inhaled.
The only downside to water is that initial dousing when a lot of steam and splatter errupts which could be dangerous to whomever is too close to it.
Even plugged into a charger, water may be the optimal choice. The device will certainly be desteoyed. Your supply breaker should trip after a few Sparks, cutting power.
The point is: what is the thinking for trying any other method? Saved the equipment (destroyed anyway with poweder options)? Don’t start an outlet fire (GFCI should have tripped.. go to the breaker box and cut manually., Or yank the cord from the wall).
You want to get the battery fully submerged as soon a is safe to do. Water is the only easy method of accomplishing that.
We now have welding gloves, long tongs, face shield, mask, and fully body welding apron (with arm coverage) next to our charging atation. As well as several buckets waiting. We charge a lot of high power batteries (we trialed each model we use at the burn field).
Mostly sounds good, other than the breaker thing.
The danger of water on plugged in equipment is that you can get shocked, and you can be killed by way less than the 15A that would trip a standard breaker.
Water isn’t a great conductor, so it likely won’t draw enough trip the breaker at all, it’ll just sit there being an electrocution hazard. A GFCI is more likely to trip, though it’s not a sure thing (you need several mA getting to ground for it to trip).
Yeah it’s certainly not perfect advice. Maybe ‘run’ is better lol.
At the end of the day, it depends on the person, the equipment, the situation.
Douse it long enough to stop flames. Then hit the breaker or pull the cord. The douse it a lot more.
Obviously there are BETTER extinguishers for equipment fires. But most people don’t have them / can’t afford them.
As you write, water isn’t a great conductor. With normal mains voltage it is sufficient to keep at least 1m of distance. You write “15A”, so you are probably from north America with the extra low mains voltage (115V, only half as normal here) where you need even a really good contact to receive a fatal shock (100mA or more).
My Apple watch battery swelled so much that the face burst open. Then it got really warm. I yanked off my watch before the thing was going to burn. fortunately, it didn’t. But it freaked the heck out of me. Next time, Garmin.
Garmin almost certainly use the same type of batteries, and quite likely made within a few miles of the ones in Apple watches.
All these batteries can swell with age, regardless of the make of the device they’re installed in. I’ve seen it happen with HP, Apple, Samsung, IBM, and other kit. When they do, stop using them immediately, and replace them. It’s not the kit, it’s the batteries.
Friend of mine had his Apple Watch Gen-0 battery swell after a few years of use. He took it into the Apple store and they replaced it free with a series-3 watch (the oldest they had in stock) – which he gave to me, as he was upgrading anyway.
At least one model of Macbook was made to cause itself extra damage when it’s LiPo pouch cell battery went bad and inflated. The battery housing has two 3 sided flaps on top, covered with a big piece of black tape. Those flaps are directly beneath the touchpad. When the battery goes bad it puffs up under the touchpad and breaks it.
So to repair it for its owner, I had to find a replacement touchpad and get a new battery from OWC. Don’t buy the cheap batteries that don’t correctly lie to the computer about being genuine Apple. The Macbook is designed to throttle the CPU to minimum speed if it detects a non-Apple battery.
It is always good to avoid Apple products. Not so much because of the batteries but because of their “walled garden” or “golden cage” philosophy.
I do believe Tesla has done a superb job in advancing safety of Li-Ion batteries. The reasonable choice of cylindrical cells, the wire fuse to both – anode and cathode, heat resistant epoxy lamination, monolith positive and negative terminals. It really is as safe as Li-Ion will ever be.
And that’s sad, because if this is the pinnacle of li-ion safety then EVs can never become truly successful.
Cylindrical cells waste a lot of space and mass, and they cost a lot to manufacture and assemble. What relative safety advantage there is becomes moot by the fact that you don’t want to pay the price of the battery anyways.
Does that apply to Optima automotive batteries as well?
This space is not really wasted. You need channels for cooling anyway. For one solid block of battery it would be quite difficult to control the temperature and remove the heat.
As with all engineering problems there are trade offs. High performance battery packs need cooling channels. Otherwise they rapidly overheat. The slowest Model S can draw 200KW. Part of the charging cycle can suck as much as 150KW. Even with the minuscule internal resistance that adds up to a lot of heat. Active cooling, using the AC, is used to pump out as much heat a possible. That is how one achieves charging speeds of 400mph.
I had a MacBook Pro battery swell badly a number of years ago, to the point where it popped the casing open.
I had a similar MacBook Pro experience, bought a replacement battery because I’m an adult. Tesla is doing great work, the batteries that haven’t burst into flames should be okay… until they burst into flames, then you’re being pursued by a self-driving car on fire. Skynet.
Something puzzled me about Lipo packs used in the model industry, especially considering that multi cell ones have a separate balance connector: how come there is no temperature sensor attached to the battery?
LI doesn’t typically get warm under (did)charge, and if it does it’s too late to do anything electrically
My Li tool batteries, 18650 cells in the chain saw pack, 60Wh or 100Wh, discharged in 15 to 20 min of work, regularly get warm under discharge. They are warm enough that the charger refuses to charge them before they had time to cool down.
video does not working. can someone rip it and post it to youtube?
The video _is_ on Youtube. Here is a non-embedded link: https://youtu.be/BLc74Qpvweg