At some point, the 3.6 V of a single lithium ion battery just won’t do, and you’ll absolutely want to stack LiIon cells in series. When you need high power, you’ve either got to increase voltage or current, and currents above say 10 A require significantly beefed up components. This is how you’re able to charge your laptop from your USB-C powerbank, for instance.
Or maybe you just need higher voltages, and don’t feel like using a step-up converter, which brings along with it some level of inefficiency. Whatever your reasons, it’s time to put some cells into series.
Notation Confusion
The common notation for battery packs in parallel or series is XsYp – as in, the battery consists of X cell “stages” in series, where each stage consists of Y cells in parallel. So, putting three cells in series is 3s1p, a single cell is technically 1s1p, and two cells in parallel is 1s2p.
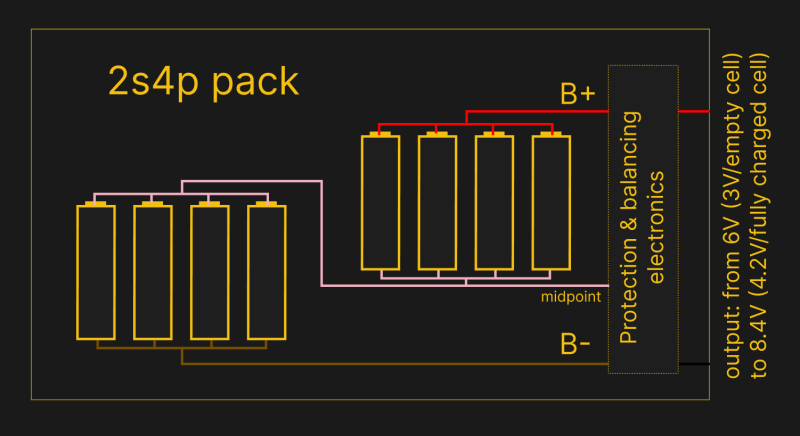
A less precise but more popular notation is just showing the pack voltage – either the final charge voltage (4.1 V to 4.3 V) or the nominal voltage (3.6 V to 3.8 V) of a single cell, multiplied by the amount of series stages. For instance, if you see 12.6 V pack voltage, it’s exceptionally likely to be a 3s?p pack (4.2*3), and if it’s 14.4 V, it’s likely a 4s?p pack (3.6*4). This notation is less precise, because you don’t know the number of cells and you have to deduce cell voltage was used for calculations, but it’s the one that’s most often actually put on product and battery labels.

This obviously becomes a problem when you’re working with packs like ebike batteries. What does 52 V represent? Is it, 14 3.7 V nominal stages, or is it 12 4.3 V full-charge stages? If that’s the value you see on the multimeter, is it a fully charged 12s pack, or a fully discharged 15s-17s pack? Are you comfortable taking a guess? I wouldn’t be.
For charging and tailoring the electronics used for the battery, knowing the cell count matters a lot. So, if your battery simply states a voltage on the label, you will have to deduce the cell count before you can charge it. If you can, it might be worth to open the battery (very, very carefully) enough that you can physically count the cells and maybe even figure out the cell connections – if you can see that the pack has four-cells groups connected in parallel and you can count 60 cells in total, you have everything you need to understand it’s a 15s4p pack.
How Much Do You Really Get?
What about total capacity? Let’s talk that through. You will see packs use either milliamp-hours (mAh) or watt-hours (Wh) for capacity notation. When you see mAh, this is capacity per stage – the sum of capacity of all parallel cells in a single stage. Wh is the per-stage mAh multiplied by total pack voltage – typically the nominal voltage.

There’s one notable exception in mAh notation talking about stages – powerbanks. Even if your powerbank has a 2s2p configuration, they will sum up mAh capacity of all cells and put that number on the label. This number is exactly twice the actual mAh capacity of the battery, though. mAh are a primary figure for powerbank capacity marketing.
So here’s a summary for working with unfamiliar packs. If you see that a pack says 20 Wh, you don’t actually know anything about it, much less enough to charge it, but it should be easy to figure out. If you see that the pack is 120 Wh, you still don’t know enough, but at least now you know that it can’t be taken on a plane. Figure out the pack voltage and which kind it is – charging the battery fully and measuring the voltage should do it. Then, deduce the battery internal configuration and per-stage capacity, write them down, and maybe even put them on a sticker glued into the – if the manufacturer hasn’t done it for you, someone has to do a proper job.
If you’re designing something that can be taken on a plane, the 100 Wh limit has workarounds that you should be able to easily deduce from reading airport battery rules – I have taken flights while carrying approximately 300 Wh of batteries in my carry-on luggage. You, too, can pull such cool things off if you know enough about LiIon packs – so let’s continue learning! How do you build your own pack? Let’s wrap up by talking about cell selection.
Pick Your Cells Carefully
If you’ve ever taken old laptop batteries apart, you might already have an assortment of salvaged 18650 cells, and maybe you’ve even got few cell holders handy – if not, you should. If your plan is to be putting these cells in series, however, watch out – you can’t use any random cells.

For every cell, you need to know two parameters – cell capacity and cell internal resistance. Those two need to at least approximately match if you’re building a pack. Naturally, if you combine cells from different sources, they’re not going to match well. However, you can still find cells that are good enough for your use! What happens if you don’t?
What Could Go Wrong?
Let’s look at a single parallel stage for a start. If you mismatch capacity between cells within a stage, then the stage capacity will be limited by the capacity of the cell with the lowest capacity. As soon as the lowest capacity cell’s energy is depleted, the cell’s voltage will sharply drop below safe voltage, and the cell will be charged from the other cells around it in quite unfavourable circumstances.

In the same parallel stage, if you mismatch the internal resistance within a stage, the lower-resistance cells will take more charge/discharge current than the others, increasing the load on it beyond what you would intend to put. Oh, and it’s going to heat up more, which will negatively impact its capacity/internal resistance, and heat up the cells it too, to the same effect. Both of these problems can cause the entire parallel stage to die with the cell affected – which is something you often see happen in laptops.
What about differences between stages, within the series configuration of the pack? If a stage has higher internal resistance than the others, it is going to heat up more, and it is going to drop more voltage – causing an annoying dip as soon as you draw current from the pack, and increased balancing complexity during charging. This, again, is a common fault in laptops and powerbanks, as well as e-bike batteries. As for capacity, a stage with lower capacity than other stages will limit the capacity of the entire bank, causing the same kind of dip. Not only that, but if you continue drawing energy from the pack after a stage’s capacity has been depleted, you’re going to be charging the drained stage in reverse – with potentially fiery results.
As you might notice, the mismatched internal resistance and capacity problems are similar, and that’s because internal resistance and capacity are tightly interlinked parameters of a LiIon cell. Fortunately, they’re easy to assess.
Assess, Configure, Equip
The simplest way to determine cell capacity is to get a cell tester. Those tend to be able to discharge and charge individual cells while also measuring parameters like capacity and internal resistance, and logging the results. There are quite a few cell testers, and it’s a popular project in the DIY scene too. You can test pouch cells the same way you would test 18650s, of course, just that it might require alligator clips or adapters.
 Failing that, you can intuit cell capacity and internal resistance with a couple of high-power resistors – basically what the testers do. After charging a cell fully, measure its voltage, then start discharging a known-value resistor through it, measuring the voltage drop. Comparing this voltage drop between cells, especially at different points in time, will give you a proxy for the cell’s internal resistance.
Failing that, you can intuit cell capacity and internal resistance with a couple of high-power resistors – basically what the testers do. After charging a cell fully, measure its voltage, then start discharging a known-value resistor through it, measuring the voltage drop. Comparing this voltage drop between cells, especially at different points in time, will give you a proxy for the cell’s internal resistance.
As for capacity, you can use a TP4056 charger to fully charge your cell and then use charging time as a proxy for capacity – you might not be able to differentiate a 3200 mAh cell and a 3100 mAh cell, but you’ll definitely notice a 2500 mAh cell standing out. Alternatively, you can use a resistor and note discharging time until reaching a certain voltage – while you can’t estimate capacity unless you’re also constantly measuring voltage, a rough estimate of discharge time will do.
Plus, take note of cell heating! If a cell is heating up beyond norm during charge or discharge, again, consider recycling it. Cell testers and chargers tend to measure temperature for this exact reason. Again, buying a bunch of cells from the same batch is the safest thing you could do, but if you can’t avoid combining cells, now you know what it takes to do so relatively safely, and what are the specific ways it can go wrong.
Today, let’s stop here, and next week, explain all the practical aspects of pack building and reuse. If you want to build a LiIon pack, which configuration do you pick? How do you charge it? What kind of protective electronics do you need? What else to watch out for? And what even is balancing? All of this and more will be explained the next week, and I hope this article can help.

















Nice write up!. This is one of those areas of engineering that only engineers should be doing, not curious guys on the internet; too dangerous and expensive to be playing around.
Thank you! And, absolutely disagree – the reason I wrote this article, is so that people can hack on things safely. LiIon packs have a lot of power to them in multiple ways, and I believe hackers should be able to harness this power, while knowing about the possible pitfalls.
May I humbly suggest LiFePO4 batteries. Nominally 3.2v (3.0-3.6v) they have many benefits and few down-sides.
Benefits:
Their voltage aligns better with devices using alkaline or Lead acid than Lithium does. EG 2S is 6.4v (2x Alkaline) and 4S is 12.8v (“Car” battery).
It takes a little bit of float charge without thermal runaway, unlike Lithium.
It is measured in thousands of charge/discharge cycles. Like 2,000-5,000. Li-Ion is typically 500 cycles.
Negatives:
A little bigger and heavier, typically only available as a 26650 cell.
For a C cell replacement, or Lead acid seems very well suited.
Not detracting from Lithium, I do wish heartily the TP4056 module had settings for 4.1 or 4.0, 4.15 volts so that we could get a useful lifespan from these devices instead of destroying the battery with top charging needlessly every time. Very sad.
Looking at the curves you will see keeping the Li-Ion battery between 20-90% greatly extends the life span. 30-80% even more so. Most devices you would likely put the battery in can simply have a larger battery to make up the difference in useful capacity. And benefit is the battery is less likely to be damaged when left discharged for long periods.
They are wonderful, from what I’ve heard! I just haven’t had the chance to play with them much, yet, ngl – have had good results with Li-Ion so far, and buying a bunch of different cells then changing all my tech to fit them feels, unnecessary? I have a toolkit for Li-Ion, not so much for LiFePO4, sadly. Also, I’d really need 18650 style LiFePO4 cells. That said, I am making a note for the next time I’m doing an nkon run!
Also yes on the TP4056 not having 4.0-4.15V thresholds, it is a bit sad! I’ve now found CN3305 which is a configurable threshold buck charger that appears to be able to do 1S, so, I might just mention it in one of the upcoming articles/
Friendly correction:
Alkaline cells are normally 1.5-1.6V per, and 1.2V for rechargeables.
While I admit I mistakenly put the voltage of 4x Alkaline instead of 2x. My point was any device that takes a multiple of 2 Alkalines will be fairly happy with replacing every pair of Alkalines with a single LiFePO4. (well, happier than doing the same with a 3.7v Li-Ion.)
Also Lead acid is nominally 2.1v per cell, if you get a 6v 3 cell, or an 8v 4 cell. Even 7 cell “14v” are available for race cars that don’t have a charging system. The voltage drop under load puts them in a similar range as a normal 6-cell that is being charged by an alternator.
Not to mention 1.7v AA lithium primary cells (No idea how they do that)
The main downside of Iron vs Cobalt (separator, assuming that’s what you mean by lithium above) is the energy density being lower. The weight’s only higher because it takes more mass to equal the amount of energy stored.
Auto’s using Manganese too these days (had to go refresh my memory on what those cells were) and that might be in other applications as well.
Although to be honest I wasn’t sure what metallurgy the article is about either. “Lithium Ion” encompasses iron and all the other variants of lithium chemistry as far as I know. I’m not sure cells from power tools are Cobalt specifically anymore.
i am using them in my 3.3v projects most of the time. They seem a good power match.
I’d take a position mid-way between you and Clovis. Hack with Li packs, sure, but do it outside, and never bring the results in to your home or any other building.
Also, do batteries of any type (NiMH, Alkaline…) ever get put in parallel in any product? I think not, when a product needs a high current they stack up more cells in series to give an excessive voltage, then use a step down converter (buck of some sort) to provide a regulated high current and some regulated lower voltage.
Actually I’ve but batteries in parallel … in my R/C airplanes. Same voltage, but more capacity for longer run time. Put to packs in parallel is easy. Just more weight of course… Can’t put in series as then you’d have to change props (or motor) due to RPM/V issues causing excessive current. A balancing act :) .
Which brings up the LiFe … Weight is the problem compared to LiPo batteries. And not sure about how much current you can pull from the packs. I’ve not seen anyone use LiFe in model airplanes except for powering receivers in the gas planes.
trade off for lower fire risk (thermal runaway) is lower nominal voltage, about 3.2V IIRC, and thus lower output in the same size battery (i think I’ve seen 1800mah cells). So maybe not a good trade off for RC stuff (more weight for same power), but a good choice to power a cabin in the woods! (400 Ah battery pack 10′ from me as I write this on the laptop – though I bought them as 4 car sized batteries)
I’m sorry, but that’s very much not representative of actual failure rates of self-built Li packs, to the point where it makes me question if you happen to own multiple properties that you rent out ;-P And that’s under an article which discusses Li pack building and usage safety in detail, which misses the whole point.
Why should your stance be taken seriously? What is the justification for your fear? Got numbers to back it up?
Li cells in parallel are very much the norm in laptops *and* e-personal mobility stuff *and* powerbanks etc.
Some high power flashlights put 3-4 high current cells in parallel, in order to minimize internal resistance so they can dump 20+ amps at 3-4 volts through a mosfet into the LED array. You just make sure to use a set of identical cells and never mismatch them. No BMS is needed because there’s no series connections, so when the voltage gets low enough you recharge all cells to the same final state of charge, but you can do it in individual bays as long as there’s no significant difference in voltage when you put them back in parallel.
Engineer = someone else pays for you to play around with expensive and dangerous things
As more and more devices become all electric (i.e., cars), those lowly non-engineer types will need to be able to interact with LiPos and understand the basics.
Having worked on commercial aircraft with massive LiPo batteries my advice would be to start small and work your way up. Adafruit has some small lipo boards that you can connect with a 3.7V ‘pouch’; you have to really try to hurt yourself with that.
Questions…
Do any commercial aircraft really use LiPo (Lithium Polymer) batteries? That seems … ill advised.
Search for Dreamliner battery fire
>> “Engineer = someone else pays for you to play around with expensive and dangerous things”
You… say that like it’s a bad thing….
It only takes a small spark to start a large fire…
Curious girls however… They’re fine! :)
couldn’t have said it better 😊
What I meant with the text above is: this is not something the general population should be playing with, e.g a random person trying to make its own electric scooter and causing such batteries to catch fire. We have to remember that these batteries have an increasing energy density, it can kill somebody for sure
Lower energy density than gasoline – general population has been interacting with that for a while
I don’t know anyone who would carry a gasoline filled device in their pocket.
Errrr ……. a cigarette lighter?
By extension, then people should not be allowed to play with gasoline, gas stoves, candles, or even matches or firewood, as they all have energy density far in excess of a battery. Heck, even a butane lighter has more energy density than a lithium cell, and arguably more dangerous.
There’s one distinct difference: a fuel fire can be put out by smothering it. A lithium battery fire, not so easily, it gets angry if you try to douse it with water, and it re-ignites spontaneously.
That’s only true of certain lithium battery chemistries. Lithium-Iron chemistries, for example, do not provide their own oxidizer, nor are they exothermic upon exposure to water.
I don’t think anyone should be causing their packs to catch fire either, that’s why I’m writing about what exactly happens if you screw things up, so that people know what to avoid =D Knowledge is power!
You remind me of factory management, treating engineers like gods despite some truly questionable designs leading to long production times and lost contracts.
Looking shit up for cad / ee isn’t that hard dude
If you are building an E-Bike pack I would heartily suggest sourcing an EV battery module instead of building up a little 18650 pack. A Hybrid battery module will deliver more current and charge faster, but have a reduced range. It may be preferable if you have a high-draw system and EV packs are likely 30-100Ah anyway, so range is not likely to be very poor anyway.
If you take a standard 50Ah 40v (36v nominal) 10s EV pack it might cost ~$300 (Prices vary a lot, and Pick n Pull salvage yard is now listing $400 for an entire EV battery pack, enough for several e-bike packs). You may cringe. But it will likely be pre-wired for BMS, have a heavy duty shell, high amperage interconnects (sometimes with bolts). Once you add all this to an 18650 traction pack the used EV modules can look very attractively priced, if not cheaper.
I might also suggest a 900w and up DC DC current and voltage limited power board attached to a recycled server power supply or 24v 5A-10A power brick as an inexpensive way to trickle charge packs up to 100v. ($20 for the DC-DC, $3-10 for an AC-DC Brick, $10-40 for Server or Meanwell PSU)
“As more and more devices become all electric (i.e., cars), those lowly non-engineer types will need to be able to interact with LiPos…”
Very, very bad idea because a mistake can easily kill you or cause a very ugly catastrophe.
Tesla: the 100kWh battery on the Model S is 400 volts. This battery includes 16 modules with 6 groups of 86 cells, which makes 516 cells per module. Each of the 16 models produces 25 volts, giving you a final total of 400 volts.
For EV, the old warning should definitely be heeded:
Do Not Open—No User Serviceable Parts Inside
I guess we should prevent people from working on carburetors, fuel inected cars and even pumping their own gas because a mistake can easily kill you or cause a very ugly catastrophe.
None of those are “passively lethal” like a high voltage battery, where simply touching the wrong part will instantly electrocute you.
suuuure =D nevermind the potential for explosions during storage?
one hand in pocket, easy 😝 I’m going to be talking about this in the next article – my understanding is, even ebike packs can be seriously dangerous to your health if your hands are sweaty enough!
There are several people out there servicing EV battery packs, just need to take precautions – Discharge and wear proper PPE.
I would make your same argument about people rewiring houses.
A very good article: very much needed; superbly-written; extremely informative; author very obviously knows the subject well.
We need more such as this.
Excellent article, Arya!
After watching Big Clive’s video on E-Cigarettes (Vapes)I have been collecting them and extracting the rechargeable cells to make battery packs. I have around 200+ of various types.
http://www.sadarc.org/xenforo/upload/index.php?threads/make-a-battery-bank-for-almost-0.362/
So far, I have enough of one sort tested to make a 45AH 12V battery if I want. As I also have a “Granny Scooter” here, I may end up using these cells to make up a 48V battery for it.
These cells are 18450 , 3.7V 5.180Wh, type and around 1.4AH by my tester.
It is really bad these are single use devices, ignoring the health risks, just a waste tossing them out. A couple of days ago, I used one of the packs I’d made to run my hammer drill to mount some fire extinguishers on a brick wall. They are also used to run my ham radios. As long as you use a correct charge controller board, these are well worth rescuing from land fill.
> Even if your powerbank has a 2s2p configuration, they will sum up mAh capacity of all cells and put that number on the label. This number is exactly twice the actual mAh capacity of the battery
Summing up the mAh capacity of the cells is a legit way of doing it, because in a power bank you have a buck-boost converter at the output that will translate current to voltage and vice versa. Actually, it’s converting energy (Watt-hours) to some combination of current and voltage, and energy is the sum of the Watt-hour capacity of all the cells.
In other words, if you were to set the output voltage of the buck-boost converter to equal the voltage of one “stage”, you would indeed get twice the milliamp-hours out by adding another stage in series, even though the actual charge capacity does not double.
Of course it’s not 100% efficient and it varies depending on the load, but it gives you a useful common reference point, because the usual application of power banks is charging cellphones with a single cell in them, so the voltage is stepped down by buck converters that do exactly as described. Therefore, having a 5000 mAh phone battery and a 2s2p set of 5000 mAh cells in the power bank for “10,000 mAh” capacity actually does translate to almost four full charges of the phone – not two. The manufacturer may have also already de-rated the bank for the expected output rather than simply adding up nominal cell capacities.
It’s _not_ legit if they’re telling you that it’s a 10,000 mAh power bank with a USB 5 V output. That’s a 38% exaggeration.
I see your point though. If you’re charging another 3.6 V battery with the power bank, you’re going to convert the voltage back down anyway, so except for the losses in the up/down conversion, it’s ballpark right. Should they say “charges up to 10,000 mAh worth of 3.6 V single-cell batteries”? Probably, but I also see why they don’t.
It would be simpler if everyone used watt*hours. And the numbers would be bigger too!
Arya’s point is that for a project of your own, you can’t expect 10,000 mAh at 5 V output, because that’s not what you get. And because of boost converter losses, you don’t even get 10,000 * 3.6 wh, but that’s probably pretty close for small current loads.
It’s a problem when you want to figure out the power budget for a project that isn’t just charging up one battery from another. (When I say it that way, it sounds really dumb. Cell phones should have replaceable batteries.)
And to be totally fair, she originally waded a bit deeper into those waters, but I cut some out in editing because I thought it was going too far astray.
>That’s a 38% exaggeration.
Try and see. Maybe it does put out 10,000 mAh at 5 Volts, and if it doesn’t then you can claim false advertising and sue them for money.
For a different point, you can’t expect to drain the full capacity out of a lithium battery anyhow except by slamming it full to 4.2 V and draining it down to 2.5 V which kills the battery in a few hundred cycles. There’s technically no upper limit to how high you can charge a lithium cell, except when it catches fire, so the precise capacity of the cell is somewhat arbitrary.
For an example, I’m looking at a Panasonic CR18650GA cell, which drops from 3400 mAh to 2000 mAh in just 500 cycles under such torture. If you limit it to between 3.0 – 4.1 Volts it will last thousands, but the useful capacity is reduced by about 20%.
In contrast, my LG made laptop battery is 4.05 Ah while the de-rated capacity is 3.88 Ah or 95%. It’s driven pretty hard because even 5% more capacity is half an hour of extra run time. This also means they go flat in 3-4 years unless you further limit the charging threshold.
That’s another reason why you can’t simply add up cell capacities and call it a day. How are you going to use it? If a manufacturer of a battery pack is simply adding up the cells, the numbers are going to be wildly off, by as much as 50% in actual use – not because of the series-parallel issue.
Can’t agree. By that logic, you can’t mark a cell with a capacity number either, because a single cell’s capacity, too, depends on how you use it, just like an entire pack’s capacity does.
The pack needs to be rated for the rate of discharge, depth of discharge and expected life-span all together. If you are racing Drones you fully expect to bake a pack. Same if you are using a cordless circular saw with the 1s pack (1.5Ah, or 2Ah).
So I see his point. One number doesn’t tell me a whole lot though. And it’s perfectly reasonable for a pack to be labeled as less watt/hours than the individual cells, if the BMS prevents them from using the extra capacity in practice to extend life-span.
Although you can easily apply the 50% rule of thumb de-rating that EG GPU manufacturers give for their PSU recommendations. (150w Nominal GPU wants a 650w PSU)
> By that logic, you can’t mark a cell with a capacity number either
You can always do whatever you want. It’s just that any number you put down is going to be somewhat meaningless.
Plus, in any series connected pack, your capacity is actually defined by the weakest cell, since the BMS cannot arbitrarily keep discharging the other cells with one cell already empty.
“…And to be totally fair, she originally waded a bit deeper into those waters, but I cut some out in editing because I thought it was going too far astray.”
Based on the excellence of what what did not get edited out, perhaps a follow-on article of the edited portion is very much in order.
———————————-
“If you look at any list of great modern writers such as Ernest Hemingway, William Faulkner, and F. Scott Fitzgerald, you’ll notice two things about them:
1. They all had editors.
2. They are all dead.
Thus we can draw the scientific conclusion that editors are fatal.”
–Dave Barry
—————————————-
Just kidding; absolutely.
Arya Voronova and you are doing great jobs. Keep it up.
>It’s a problem when you want to figure out the power budget for a project that isn’t just charging up one battery from another.
You need to know what you’re doing to figure out your power budget anyhow. If you’re just mashing a power bank into a USB powered device for a project, the numbers are going to be almost meaningless. It’s only useful for relative comparison between different power banks (e.g. mAh/$). The rest you have to measure to know.
>Arya’s point is that for a project of your own, you can’t expect 10,000 mAh at 5 V output, because that’s not what you get.
You don’t know that you won’t. It all depends. There are no simple rules of thumb, because the real capacity of a lithium cell is not well defined. However, if you go to review and testing sites and look for power banks, you get measured outputs that are typically between 80-90% of what was claimed, which is to be expected given the efficiency of a typical buck-boost converter.
And if you’re building a battery pack yourself and make the mistake of summing up charge capacities in series, then the problem is that you don’t know what you’re doing.
Nice article! One typo though (I think). “The common notation for battery packs in parallel or series is XpYs “, but every example in the text is XsYp. I assume series, parallel is the actual convention?
oh yes, thank you, fixed! indeed, XsYp is the convention
Nice article! Thanks for all of the useful info.
I question the first paragraph in the section “What could go wrong”. The parallel cells in a stage will always have the same voltage. There is no reason one cell would ever be charged from the others (unless they have different voltages when first connected in parallel). During pack discharge, some cells in the stage may be able to supply more current than others as their (all the same) voltage drops, and the capacity of the stage will depend on the sum of the current supplied by each parallel cell in that stage.
On the other hand, expanding on what Dude wrote, the weakest stage will limit the capacity of a _series_ connected pack, since that stage’s voltage (and consequently the pack voltage) will rapidly drop once it is fully discharged. And since you don’t want to over-discharge any stage, you should stop discharging the pack as soon as any stage voltage drops too low, even if other stages have remaining capacity.