Researchers have been testing a new type of lithium ion battery that uses single-crystal electrodes. Over several years, they’ve found that the technology could keep 80% of its capacity after 20,000 charge and discharge cycles. For reference, a conventional cell reaches 80% after about 2,400 cycles.
The researchers say that the number of cycles would be equivalent to driving about 8 million kilometers in an electric vehicle. This is within striking distance of having the battery last longer than the other parts of the vehicle. The researchers employed synchrotron x-ray diffraction to study the wear on the electrodes. One interesting result is that after use, the single-crystal electrode showed very little degradation. According to reports, the batteries are already in production and they expect to see them used more often in the near future.
The technology shows promise, too, for other demanding battery applications like grid storage. Of course, better batteries are always welcome, although it is hard to tell which new technologies will catch on and which will be forgotten.
There are many researchers working on making better batteries. Even AI is getting into the act.















I’m immune to positive news about batteries. Until it leaves the lab and becomes economically feasible to produce it’s not real. So far improvements have been small incremental changes, not large leaps.
Batteries are hard. You want high energy density, high power density(discharge current), fast charging (charge current), high roundtrip efficiency, low self discharge, long life (cycles), can survive temperature extremes, cheap, safe,… New technology gives us mostly new tradeoffs, but rarely overall improvements (those take a lot of time to invent and perfect).
Second that. “I don’t believe anything, if there is no video!”.
Seeing is believing, except for deepfakes.
Unfortunately “seeing is believing” is still true in the latter case; otherwise they would be no purpose
“According to reports, the batteries are already in production and they expect to see them used more often in the near future.”
You’re welcome.
I didn’t think @C’s remarks justified a snarky reply.
Where, exactly, are these batteries that last 10x as long? I don’t know anyone who doesn’t gripe about cellphone battery life, for example, so why isn’t Samsung or Apple crushing the market with an ultra-long-life
Phone?
“In production” doesn’t necessarily mean “available at Digikey” and might instead mean “available to space and military users with deep pockets that only a tax-payer could fill.”
If the latter, then for the average Joe, they’re still basically unicorns.
Yeah, I totally side with C’s, there isn’t a single week about news about a totally new battery revolution incoming… but they never materialize or are vastly inferior to what was announced and just minor welcomed improvement…
If they made a phone battery that would last 10years nobody would buy a new phone. These companies dont want you to have a battery that lasts that long and they will do everything in thier power to see that you don’t.
Exactly. That’s what I meant by tradeoffs. These batteries might have 10x cycles, but might also cost 10x.
If all battery news was true we would have batteries with 10x on all specs for the same price by now.
Also where’s the hack? I rather see an article about pulse charging to extend battery life.
“Already in production and they expect to see them used more often in the near future”
-The people working on nuclear fusion, probably
“I’m immune to positive news about batteries. Until it leaves the lab and becomes economically feasible”
The article isn’t actually covering what’s going on here very well: the interesting thing about that paper isn’t the single-crystal cells, it’s that they’re actually able to spatially image commercial cells non-destructively using X-ray diffraction. (The image here is a bit misleading, it’s an overlay of the XRD data with a CT image).
Single-crystal cells are going to take a while, but being able to confirm that they’re actually mitigating what’s going on is important. There’s a big difference between studying a cell manufactured for an experiment in a lab and a cell you just go and buy.
It’s really, really hard to study lithium-ion cell aging.
I think you are right. It looks like new and very useful techniques to study battery degradation. These techniques might result in somewhat better batteries in 5-10 years time.
i have the same mentality but also
these incremental changes are not immaterial. and in fact a lot of stuff is shipped and in use that i am only vaguely aware of. a lot of things went from premature press release to finished product without a lot of fanfare in between.
and of course a side effect of that is, it’s easy to not have exactly what you think you have. the cells inside a dewalt cordless drill pack are a subtly different ‘lithium ion’ technology than the ones in your laptop or in your nicotine vape or in your radio control airplane. there’s a huge diversity of these different kinds of lithium battery now.
“I’m immune to positive news about batteries”
Same here. A few years ago there was a story about a battery that was recharged simply by changing the liquid electrolyte that could be handled by existing gasoline infrastructure. At the gas station, the spent electrolyte would be pumped out and fresh pumped in – very quick recharging. The only changes needed to a gas station would be spent electrolyte holding tanks and changes in the pumping system. The spent electrolyte was sent back to a facility to chemically change it back to a useful state. Haven’t heard about it since.
Real ones are being deployed: https://en.wikipedia.org/wiki/Flow_battery
Though it looks like they are used more for fixed grid storage rather than transport.
This battery sounds good they degrade by that separator moving larger and smaller wider and not so wide and it degrades the separator when you make that separator out of a harder substance it can’t do that and I guess that’s what they’re looking for now. Yes it’ll be great when you can actually buy this type of battery that they say could last a hundred years. It’s junk that they put the cell phones batteries in there so you can’t open them and the battery degrades and you have to get a new one they wouldn’t like that if you didn’t have to get a new one all the time.
The battery issues are related to it’s name.
Since old times people were shouting: “Battery, fire!” and that kinds stuck. Also it kinda sucks, but we’re supposely working on it.
Better start thinking for a new, cool name.
To start this naming contest I’ll start easy: charge trap (“electron trap” would cull the ideas that might use holes as things stored). From this comes the following idea: what if we rip electrons from the inner orbitals also?
They are called “batteries” wrong as they are accumulators. Just once it enters the (American) English language it sticks.
They are lithium soul crystals. Ethereal cores. Phlogiston tanks. Whatever. Is it capacitor or condenser? Was “condenser” the proper queen’s English and capacitor the American bastardization? A rose by any other name still catches on fire if you use a cheap BMC?
Designed impositions upon language of this style basically never work. It’s called a battery. Just be glad they didn’t name it assault
Your teachers, with their arm wavy science…they didn’t understand, failed you.
‘Ignition!’ (John Clark) is a good book, if you’re into chemical energy.
No empty lower electron shells though.
They are called batteries because there is a battery of cells.
Just like artillery had a battery of guns, lined up in a row.
Live with the name.
Perhaps German.
They love spannungstromzeithaltordnen type words.
You might be able to convince them.
Exactly, I fail to see how “battery” is an inappropriate analogy yet “trap” is not. They are both word-metaphors comparing the thing to something else. One refers to the cells lined up in a row like a battery of canon, the other to the ability to contain electrons or charge like catching a bear in a set of iron jaws. What’s the metric here for which is good and which is bad?
Nearly all of etymology is like this, going back to and beyond proto Indo-European roots.
Take for instance terms for ancient technologies which all have the same names everywhere, owing to the sheer age of the root words. Ashlu, “axle,” refers to a man’s shoulder. Hwewlaz, “wheel,” refers of course to the shape of a circle, but also interestingly the habit of ambling about while staying in one place. To dwell on something, I.E. the habit of a wheel for spinning about a single point.
Upon further reflection and observing the screenname, I think that perhaps their concern is simply based on an aversion to any talk of guns and weapons. But a “trap” is still a weapon of sorts anyway, so..
“Firefly jar” sounds better my good sir?
Cars have been having the same issue for a long time. Even ICE cars have become so reliable (with proper maintenance), and the car weak spots are always the gremlins in connectors and rust. Having a battery that can drive you 8 million km is not very useful when the car falls apart after 200k km.
I understand that it goes against the interest of car sales, but some more effort should be put into making the cars last longer.
“when the car falls apart after 200k km.”
200k kilometers?? That’s ~120k miles! What the heck cars are you people buying?
Most typical car issues nowadays are either just stupidity (poor maintenance, and rust is a maintenance issue) or cheapo design issues.
The battery issues are more complicated than just pure mileage (cycle count) since batteries don’t just age from cycling, they also calendar age. But the main issue with batteries isn’t their longevity, it’s replaceability.
It doesn’t even matter if cell prices keep dropping if the manufacturer changes batteries every few years and golly gee, your old battery isn’t manufactured anymore, sorry, there’s nothing we can do. EVs are godsends for car manufacturers: so long as they just make it so that each generation’s charging system is somewhat bespoke, they’re not going to be economically fixable.
“Sealed automatic transmission fluid is rated for the lifetime of the vehicle.”
Translation: the vehicle dies when the trans fluid goes sour and there’s no simple way to drain it out and refill it without significant disassembly, so nobody does it. Car totals with the transmission. Sometimes begins around 60k, usually only presenting serious challenges and failures to the driver around 120k as the internal clutches glaze and start slipping.
Entirely and easily preventable, smart drivers will find a way to open the “sealed” transmission and change it on a regular service schedule anyway but this type of driver is unfortunately very rare and getting rarer.
“but this type of driver is unfortunately very rare and getting rarer.”
Yup. That’s the correct answer. I’m a crap mechanic compared to my family, but so many people I know look at me like I’m a friggin’ genius, and it’s scary.
Another factor is that your car may be the only car of the household, so keeping it out of service for doing such operations can be a no-go. If problems develop, instead of spending weeks and months to diagnose and fix it, people just drive to the dealership and pick another second hand car for not much more money.
You’ve never lived in the salt/rust belt.
You can’t maintain around it, rust just happens.
Nice car stays in garage for winter is only option.
Have ‘Paris car’, one that’s already rusted and dented on all 4 corners and sides.
But yeah, the only cars rusting in half at 120k aren’t driven any miles/year.
I moved to warmer climate.
Most typical car issues these days are garbage from the factory.
New car buyers are just stupid.
jatco CVTs, EcoTechs, 4 banger turbo full size trucks, Nissans, VWs, Fiats, Benzes…They shouldn’t be able to give these things away.
The maintenance cost still makes them worthless.
Just pinhead MBA math and decades of optimizing status symbol value.
Hint:
They became status symbols by being good cars first.
Making them better status symbols by making them unaffordable to maintain past second owner is bold choice.
An additional problem is that it is now nearly impossible to legally build or import any car that is not those crummy things you mentioned. They just won’t let you do it, the local authorities will force you to build it crummy and then fill the cab with 47 airbags for some reason
“You’ve never lived in the salt/rust belt.”
I do live in the rust belt, and my cars literally have been to the Moon and back.
“You can’t maintain around it, rust just happens.”
Yes, you really can! Undercoat (not the rubberized stuff) actually does work, and even if there are spots that get missed, you can repair and manage for a very long time.
Europeans drive less. The average distance covered by car in a year in the EU was just under 11,000 km in 2022, down from 13,000 km in 2000. A 200k car in the EU is probably going to be 18 years old and well ready for scrap unless someone picks it up for a fixer-upper. Even if it was kept well, it’s going to have issues of old age, like hardened seals and water ingress, cable rot, brittle plastic parts, rusted brake cylinders, etc. so it’s basically in a “replace everything” condition.
The US average in 2022 was around 21,800 km. For that sort of driving, a 200k car would be just 9 years old and probably a good catch on the second hand market.
“A 200k car in the EU is probably going to be 18 years old and well ready for scrap”
I mean, a 200k car in the EU is probably a 200k car made by a European manufacturer, so yeah, that’s different, but again, why are people buying these things? A well-maintained 2006 car with 120k miles on it being scrapped?
Seriously don’t get it. The mechanics in my family snap those cars up in five seconds.
That’s another difference: people live in apartments, not houses, so no garage for endlessly repairing your vehicle. It’s sitting outside on a detached parking lot, or on the curbside, and gets minimum maintenance because it costs €100 per hour for any small problem.
“On average, cars tend to be around the 14-year mark when they reach the crusher, and that is a figure that tends to remain the same year-on-year. ”
https://www.bmssalvage.co.uk/blog/how-old-is-the-average-scrap-car/
Another reason they point out is that Clean Air Zones impose a tax on older cars that don’t meet newer Euro Emissions regulations, so people simply get rid of them.
That’s not a car problem, that’s a society problem.
Plenty of European countries have car age distributions that are way older. Common thread? They’re poorer. Claiming that old cars aren’t worth fixing is a lie rich countries tell themselves to justify a luxury.
Or lack the environmental regulations and the population/car density to justify excluding older more polluting cars. For example, the average age of cars in Sweden is 10 years, Finland it’s 13 years. These countries are almost equal in GDP-PP, so it’s not a question of poverty – just policy. Sweden even has a higher tax rate on cars.
It’s not good business to make something that lasts a long time so this will probably be one of those ideas that gets forgotten.
Came here to say this. If the battery lasts for such a long time, how will manufacturers sell you a new device?
Same way they do now? Claim that their new hotness is super-awesome, rotate between colors/looks so that everyone can tell whether you’ve also spent money to flaunt, and get rich people to flash it in front of everyone.
If you’re someone who only has a desire to replace something when it’s worn out, you are very much in the minority. The auto and fashion industry have been doing this stuff for decades.
…Or simply write some firmware that falsely displays the battery as degraded and prevents it from fully charging after six months of use
Nah, that can get them in legal trouble, paying rich people to flash their new crap and say “hi guys, guess what I got” is way cheaper.
Fear not, the market is huge. And disposable products will make for a permanent demand.
It’s reliable as a general cynicism, but there are rare companies that build to last simply to buck the norm. Almost always a privately-held company
I’m not sure I agree with the interpretation of this paper. It’s not promoting a magical new battery construction (all the cells they were testing were commercially available units they purchased, including the single crystal one, and had been used in previous work over many years), they were doing the hard work of inspecting and characterising the kinds of degradation that cells experience after heavy use in order to build better models of how these cells work and fail at the microscopic level.
After all, you can’t improve your mousetrap if you don’t know what’s wrong with the previous one.
You are right, single-crystal NMC is commercially available for quite a while. It is actually older than the commercial polycrystalline structure. Although it shows higher cycleability it is at the same time less power performance capable. And pricier in manufacturing.
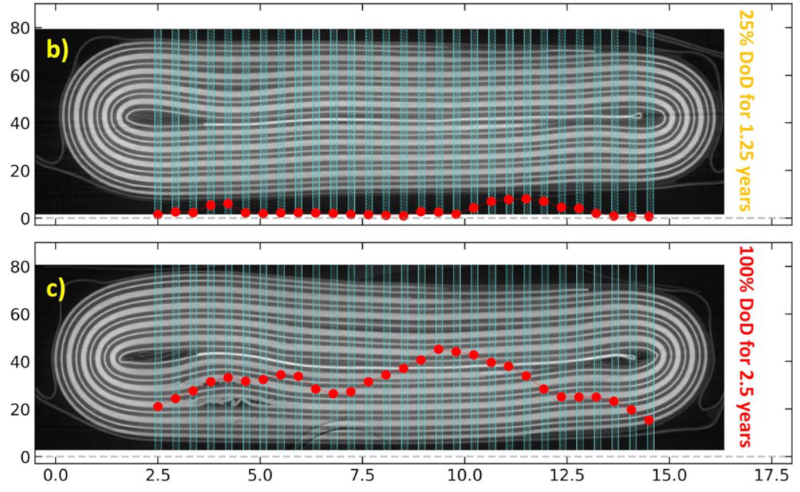
Meaningless graphs as illustration. What is DoD? No axis labels!
DoD = Depth of Discharge, a common acronym in battery testing. I don’t think it’s a graph either, it’s a cross-section of a battery pack after the usage written on the right. Not sure what the red dots are though, deformation?
The image is a CT scan of the battery after cycling overlaid with the mass loss fraction (how much lithium it’s lost) determined from X-ray diffraction over the highlighted region. The plot’s trying to show that the cell doesn’t age equally over the full cell (and that you can measure it).
Those aren’t actually the single-crystal vs. polycrystalline plots: they’re comparing a light DoD to deep DoD cell. The point of the paper is just being able to study a cell in detail while it’s in operating condition very far into its operating life, so the early part of the paper is just looking at studying aging at all. A lot of battery studies don’t actually use commercial cells because it’s Very Hard to do it.
The single-crystal vs polycrystalline is Fig 23/24, with Fig 23 being the CT scan and Fig 24 showing the XRD data. Although, as the paper noted, this isn’t a “single crystal vs polycrystalline” study, so this isn’t an apples-to-apples comparison: the paper is “look, we can study the advantages of a single-crystal cell even in commercial-type cell environments, we just need a very high luminosity synchrotron X-ray source.”
The illustration is from the paper and is properly presented there. It’s just the HaD crop for the article that removed the axis labels and presents it in a different context
In my world it means “Department of Defense.” ;)
Wrong metric. Shelf life or shelf-stability is a more important metric than cycle life, because nobody drives 8 million kilometers in a hurry.
This is especially true for long term and large scale grid energy storage applications, where the battery may only undergo ten charge cycles a year – so will the battery last 2,000 years? Unlikely.
It might be of interest to truckers? They do 100k miles a year and aren’t efficient vehicles so I’d guess they use 500 cycles a year.
Current LFP tech is a partial match for their usage, they’ll be around 80% of original capacity when a diesel truck would be having its engine replaced but a few hundred kWh of LFP will be more expensive than a lump of steel so ideally it should last longer. Weight’s an issue too, if you can get more energy dense NMC to last long enough the weight and fuel savings might make it worthwhile?
Electric trucking is already in use so it must make financial sense but anything that can swing the needle further or cover more use cases would be good.
Truck drivers may cover 400-600 miles per working day (not every day). If you over-provision the battery with a 20% margin for aging and another 20% for reserve between end points and for bad road conditions, you’re at 938 miles of capacity. Let’s call it an even 1,000 miles for a reasonable design capacity for a long haul truck. This leaves you at 50% DoD at the end of the working day, not 100% which would stress the battery unnecessarily.
That would mean you get fewer than 200 complete cycles per year, or about 2,000 in a decade – you won’t be approaching 20,000 cycles basically ever.
The NMC type of cells they’re talking about generally have a shelf-life around 12-17 years, estimated. The latest formulations haven’t been around for long enough to say for sure when they actually start to break and what happens to them over time. For the types of batteries we do have actual data on, 10 years seems to be the maximum practical lifespan – it’s not that they break entirely, but the internal resistance grows enough that it starts to cramp the power output and the BMS thinks the battery is empty when you stomp on the throttle.
This means there should be somewhere a 10 year old smartphone that was constantly used during all that 10 years and it still have a great battery that could last for two days under normal use.
Interesting, where do they get such “conventional cells”? I never met one that lasted more than 1000 cycles with more than 30%-40% left.
Or this number is obtained under some strict laboratory conditions with cell temperature stabilisation, lowest possible charge-discharge currents and so on, done on hundreds of batteries with getting rid of 99% of worst results?
I think this estimations should be taken with a huge doubt. Even if it is not a scam, then in reality you will get around 2000 cycles with 80% left. Or 5000 with 30%.
“This means there should be somewhere a 10 year old smartphone”
Smartphones don’t care about battery longevity, they don’t do anything to attempt to keep the battery cool during either charge or discharge.
And they stress the battery to the limits by over/under discharging it to gain more capacity.
I’ve had cellphones with batteries that went for 9-10 years – not smartphones though. They spent most of the time in a half-discharged state, which helps the lifespan a lot. Those batteries and all the other “light use” camera, drill, etc. batteries I’ve had for over a decade went the same way: up to 7-8 years they behave like new, then 8-9 years they start to show loss of capacity, then at 9+ years the capacity is crashing down until one day it just stops holding any charge.
Yeah, that’s rollover failure.
Bit pointless to maximize battery life, though, given replacement cycles. Plus batteries are replaceable and smartphones have real time bombs with their NAND flash anyway.
The early smartphones I had were replaced simply because they ran out of memory for anything. A simple email client is now 700 MB in size, so there’s not a lot you can fit in a 8 or 16 GB device with the operating system with upgrades eating three quarters of it.
The operating system + upgrade issues aren’t real, though, they’re due to the fact that the phones protect the factory portion of the OS in a pointless safety attempt (pointless since NAND flash has read disturb anyway and so the protection is silly).
And while a lot of the apps have grown in size to match the smartphone capacity, at least for Google there are equivalent ‘lite’ versions (the Go equivalents) which are stupidly tiny and honestly in a lot of ways better.
All in all it’s just “no one cares about smartphones lasting given replacement cycles.”
Whatever the reason, I had to start deleting everything on a 16 GB phone just to keep operating. All photos and documents and any app that would allow it was already moved to an SD card, but it didn’t help.
It’s not about smartphones, really. It is about blatant lie that “a conventional cell reaches 80% after about 2,400 cycles.”.
In real life lithium batteries show miserable performance and durability with power density magnitude orders lower than even wood. They are awful energy storage from any point of view, but somehow industries still dance around this dead end technology without any signs that this will change in observable time. And they constantly lying about real life lithium battery performance.
Interesting, that news flow about potential lithium batteries improvemets is permanent. At the same time, you hardly would recall any news about progress in DMFC area f.e. Of course, fuel cells are not perfect, but at least they have decent energy density, which is not very impressive, but is nowhere as miserable and pathetic as lithium cells one.
Perhaps if the phone had no human owner and existed in an engineer’s testbed
What size batteries are we discussing here? This can’t be a vector for high amperage discharge batteries such as those used in a BEV setup. I would expect a single crystal electrode to become a heat source when challenged with a high amp demand. And take heat source and place it with a Lithium Battery Pack. I’ll pass.
Maybe this technology is applicable to tool size batteries, but show me the devices installed in cars for 5 years of consistent use under varying conditions and I’ll listen. Until then it’s just lab hype meant to get everyone on board for the BEV folly.
Why are hybrids and PHEV technologies ignored by those hoping to cut down on emissions? They are the perfect vector for long term adoption of electric vehicles, yet all they scream when asked about this is “that’s like 2 systems in one, much more complicated….blah blah” I own 2 hybrids. My footprint has been cut significantly and the vehicles require little in the way of maintenance. But the subsidies are all for the BEV crowd and some of us refuse to join until the technology and infrastructure is fully mature.
Robomonkey, hybrids improve a small part of the problem. Yes CO2 gets a little better (going from 50 to 70mpg is less impactful than 30 to 50mpg) but they’re still emitting pollution at street level. They objectively have increased complexity over pure petrol and this shows up in the 2-3x higher chance to catch fire along with weird failure modes on some hybrid cars. I’m thinking of the BMW i3 22kWh EV which can have a 0.9L petrol engine as a range extender, only if that breaks it disables the entire car including the healthy EV side. They also don’t really need subsidising, they’re already happening. Check any manufacturer’s site in the UK and about half their range is a mild hybrid or better, good thing too as they won’t be able to sell new non-hybrids from 2030.
BEVs did need subsidising though I think we’re reaching the tail end of that. Battery density and price weren’t appropriate for mass market EVs a decade ago, they’re either close or there now thanks to Chinese manufacturing. Infrastructure for charging on long journeys wasn’t there a decade ago, it’s either close or ready now at least in the UK. The next challenge will be battery recycling, we’ll see what happens with that.
“BMW i3 22kWh EV”
Using a BMW to say “hybrids can be less reliable than pure petrol” may not be the best example. Bit like saying “modern components suck, just check out these guys I bought on Aliexpress.”
Fair point, although the i3 had lots of carbon fibre bits to save weight so I think they were trying to make a decent car. I was wrong about the engine size btw, wikipedia says 0.65L while the fuel tank was 9L.
Would you consider a Toyota Prius? Searching for ‘what happens when the hybrid battery fails’ it seems either the engine runs oddly but is fine or the car gets limited to 10mph so is useless.
If the battery fails fails, the car won’t start, since Priuses use the traction battery to start the engine.
All the other behavior’s just limp mode stuff controlled by the ECU, it’s basically the same as if a car’s ECU goes open loop in a pure ICE. (Note than Gen3 Priuses have ICE issues because they were built during Toyota’s insane flirtation with crap piston rings – this isn’t a hybrid issue, it’s the same issue they have in tons of engines from that rough time period).
With older Priuses (pre-2016 ish?) is that the batteries are readily available and affordable (yes, dealer/shop installation costs are big, that’s a completely separate problem). For older Priuses the batteries are like ~$2K-ish and they’ll pay for themselves over their life easily.
One of the best things about hybrids and batteries is that you don’t actually need lithium ion capacity: the biggest boost in efficiency comes from being able to have a smaller engine that’s running at peak torque and use the battery for extra demand. You just don’t need that much energy. Toyota didn’t even use the full capacity of the battery, they only used about 20% and kept it between 45-65% SoC!
“I would expect a single crystal electrode to become a heat source”
Why would you expect a single crystal to become a heat source and not tons of little crystals?
Li-Ion batteries currently primarily use a polycrystalline cathode consisting of an aggregate of nanometer-sized crystals, so you have tons of grain boundaries within any particle. “Single-crystal cathodes” have the cathode particles be single crystals, so there are no grain boundaries.
Polycrystalline cathodes are cheap and easy to make, but they’re extremely prone to microcracking (the aggregate breaking up), which is basically the root cause for a ton of capacity issues. Single-crystal cathodes are very resistant to cracking because there aren’t any grain boundaries for a crack to propagate through.
Also if you want a simple understanding of how cracks like this cause battery failure and why failure can be a pain in the neck, Fig 23 is a nice and easy way to view it:
https://content.cld.iop.org/journals/1945-7111/171/11/110514/revision4/jesad88a8f23_lr.jpg
All cells have excess electrolyte in them outside the electrodes. When those microcracks form, they create new voids for electrolyte to move into, and so that excess electrolyte slowly runs out. You can see that happening from the left to the right.
When the excess electrolyte completely runs out, now those electrodes end up just being dried out and functionally unusable, leading to rollover failure, which is when a battery goes from “I’m fine, I’m fine!” to “I got nothin'” in a handful of charge cycles.
Another reason is SEI growth. The layer of inactive lithium coating the electrodes grows in thickness and the pores that let the lithium ions through get narrower and narrower, until one day the molecules just don’t fit in and the battery stops charging or discharging.
It has a similar effect: “working, working… getting a bit harder… oops no voltage under load anymore.”
I mean, kindof: SEI growth/lithium plating result in inactivating portions of the electrode, but if it’s on a grain-scale it’ll be capacity fade rather than rollover failure. Rollover failure requires more cell-scale changes: so you get stuff like electrolyte loss or physical deformation due to stress causing cell-scale electrode inactivation.
So saying “electrode inactivation causes rollover failure” is backwards, it’s like “rollover failure results from cell-wide electrode inactivation.”
In the absence of stress (high temps, high charge/discharge current, etc.) it’s probably dominantly electrolyte loss. There’ve been studies where you just… add electrolyte to cells in the early stages of rollover failure and the cell regains a ton of capacity and goes back to a slow fade. But overall rollover failure’s tough to study because obviously the term is just an observation for “battery no work.”
The SEI growth leads to a similar rollover behavior.
The diffusion of the ions through the open channels or SEI pores becomes slower over the de-activated areas, which shows up as charge/discharge hysteresis under normal use. The cell appears full when it isn’t, and empty when there’s still charge left. If you charge or discharge really slowly, you can get almost the full capacity, but for all practical purposes the cell is broken. For example, my previous laptop had this issue where the battery calibration function returned the full 42 Watt-hours under minimum load, but died at 50% charge under normal load – it just suddenly went to 5% and then shut off. That was a 2018 model, developed the issue in the last year.
It has a similar self-accelerating characteristic. The “blocked” areas start out small, so the charge differences can still balance out fairly quickly and the cell works more or less normal, but as the active area gets smaller it gets stressed more by cycling and wears out faster, which leads to a faster loss of active area.
You can think of the SEI issue like having ten good cells in parallel. One cell starts to go bad and lags the others – no problem, it just works a little slower and balances out when the battery rests. Then a second cell goes bad, still not an issue, then a third – now we’re starting to see extra stress on the remaining good cells… then it’s more of a cascade failure on the whole set from that point onward.
If all the cells – or all of the electrode area in a single cell – were uniformly affected, you would see a slow “fade” of the cell instead of a sudden failure, but it’s never that perfect.
Why not just standardize and recycle existing batteries, which would achieve the same goal but with tech we already have? Nobody cares that their 12-volt starter battery needs replacing every 5 years, because these systems are already in place for it. Making something last forever with no gameplan for repairing it later is just kicking the can down the road.
We already are. Battery recycling technology is pretty mature now, even for lithium ion. It’s just a matter of getting more facilities built, which will happen progressively as the quantity of retired cells increases and the price of getting the raw materials from mining goes up.
Yeah, not so much on standardization. Lack of standardization of large-scale batteries or even the modules is a big issue.
When you pump in the electrons at same rate as gas, and batteries last for 20+ years — then I would think about an EV. For now, gas is the way to go for ‘my’ use and location. Range now seems reasonable, so can check that off the list. Anyway, good to see research is still on-going….
If the range is enough for you, save up all the money you normally spend keeping the engine running and I bet you’ll be able to afford the batteries. “♪ This four-wheel buggy is / a-dollaring me to death / for gas and oils and fluids and grease / And wires and tires and anti freeze ♪”