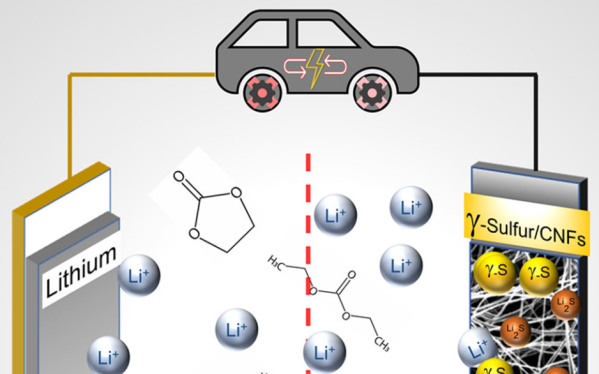
Lithium sulfur batteries are often touted as the next major chemistry for electric vehicle applications, if only their cycle life wasn’t so short. But that might be changing soon, as a group of researchers at Drexel University has developed a sulfur cathode capable of more than 4000 cycles.
Most research into the Li-S couple has used volatile ether electrolytes which severely limit the possible commercialization of the technology. The team at Drexel was able to use a carbonate electrolyte like those already well-explored for more traditional Li-ion cells by using a stabilized monoclinic γ-sulfur deposited on carbon nanofibers.
The process to create these cathodes appears less finicky than previous methods that required tight control of the porosity of the carbon host and also increases the amount of active material in the cathode by a significant margin. Analysis shows that this phase of sulfur avoids the formation of intermediate fouling polysulfides which accounts for it’s impressive cycle life. As the authors state, this is far from a commercial-ready system, but it is a major step toward the next generation of batteries.
We’ve covered the elements lithium and sulfur in depth before as well as an aluminum sulfur battery that could be big for grid storage.