Rechargeable batteries are a technology that has been with us for well over a century, and which is undergoing a huge quantity of research into improved energy density for both mobile and alternative energy projects. But the commonly used chemistries all come with their own hazards, be they chemical contamination, fire risk, or even cost due to finite resources. A HardwareX paper from a team at the University of Idaho attempts to address some of those concerns, with an open-source rechargeable battery featuring electrode chemistry involving iron on both of its sides. This has the promise of a much cheaper construction without the poisonous heavy metal of a lead-acid cell or the expense and fire hazard of a lithium one.
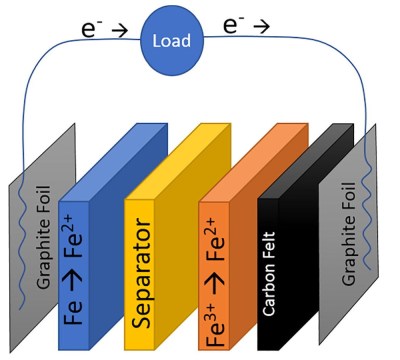
The chemistry of this cell is split into two by an ion-exchange membrane, iron (II) chloride is the electrolyte on the anode side where iron is oxidised to iron 2+ ions, and Iron (III) chloride on the cathode where iron is reduced to iron hydroxide. The result is a cell with a low potential of only abut 0.6V, but at a claimed material cost of only $0.10 per kWh Wh of stored energy. The cells will never compete on storage capacity or weight, but this cost makes them attractive for fixed installations.
It’s encouraging to see open-source projects coming through from HardwareX, we noted its launch back in 2016.
Thanks [Julien] for the tip.













Might want to correct the cost figure given: “$0.1 per watt-hour ” is $100 per kWh.
Bummer, I was ready to throw a $5 bill out there to get a whole house battery…
There is a really, really, really big difference between a battery that costs $.1 per watt-hour and one that costs $.1 per kilowatt-hour….. a factor of 1000 difference. Not a minor typo. At $100 per kWh it costs about the same as commercial lithium batteries on the cell level.
That might be 10c/kWh over its lifetime, if it can do 1000 cycles before it is considered “worn out”.
Note that the prices they quote in the paper are costs for ordering the materials and parts in small quantities.
If you can make single cells at $100/kWh by ordering chemicals from Amazon, that’s incredibly cheap because the shipping in small quantities alone costs 10x the worth of the materials.
Once economies of scale get in there it will be far, far cheaper than lithium. The ingredients are basically garbage you could find anywhere.
Do wish the world could start using a more sane measurement for electrical energy, instead of adding a second time element to a measure of power which obviously already factors in time. Instead of ten cents per Watt hour, how about ten cents for 3.6 kilojoules? I dunno, probably a futile endeavor to will that to change.
We use kWh for many things because the hassle of converting time from base 60 to base 10 outweighs the hassle of saying “kWh” instead of “j”.
The difference you speak of is comparable to that which exists between inspiration and perspiration……give perspiration a chance. Harware X is that opportunity.
Big error with the opening statement. Rechargeable battery tech has been with us for well over a millenia. See “Baghdad battery”.
They are not confirmed only theorised as batteries.
Wow. Splurge $10 on materials and you should be good-to-go, right?
Iron chlorice is a nasty material. Messy.
With any new system there are bound to be unexpected problems. In the 19th century they thought you could launch people to the moon by putting explosives under a chair. Unexpected problem: The chair isn’t strong enough, people disintegrate at the acceleration involved etc etc. Such a new battery is going to experience new problems: It’ll dissolve most containers, eat the separator. I can’t predict what will happen.
When you’ve thought of a simple, cheap way to make batteries, it is great to toss such an idea around in your battery-research-group. But make a prototype before you publish the press release.
Why are you being so condescending? Maybe the people working on this are a little more informed about it than you think. Here’s a video of one of the guys involved in the paper talking about the progress on building one including a working prototype. From a year ago.
https://www.youtube.com/watch?v=WZkqgj1J_WE
Absolutely agree, these guys have experimented, developed, and shared what they found. This is something to be positive about. There has been an on going stream of press releases making claims about the biggest best new battery or capacitor technology or use for some form of graphite/graphine/graphite that uses some proprietary mystery process that a new startup wants funding for. And your average journal article isn’t something anyone but the closest to the subject people in that field can replicate because not enough of the method is documented. It’s refreshing to see no wild claims, and highly detailed method, and the goal of making science achievable.
If you follow the link, the pdf explaining everything talks about the potential problems with materials, shows pictures of the prototypes, describes a wide variety of materials experimented with, and explains how they tested lifespan, corrosion, performance, etc. to eventually come up with their balance of cheap/safe/easy to build. They sacrificed things like surviving tilt.
It is certainly worth glancing at the prototype if you’re concerned about having prototypes.
I think you mean that their intended ancient use/ where they fit in ancient culture is unverified theory. As in, what the heck did the ancients want/do with with the unusual artifacts. -Which I would absolutely concede.
But they are the oldest know manmade electrochemical cells in the world wether they were used to “light a baghdad led” or used as part of cultural ceremonies like other pottery found in the ancient middle east.
With a name like “Bagdad battery” and with a design that operates as a cheap “rechargeable” electrochemical cell I thought there might be an honorable mention.
An O7 to the ancients
I don’t know this for a fact, I suspect no one does, but I’ve read that the speculated use of Baghdad Batteries” was to electroplate metals, probably silver, on a less expensive base metal.
1) No ancient electroplated object has ever been found
2) Examples from that region at that time show fire-gilded plating, assisted by mercury, that appear to have confused with electroplated materials by one person in the 1930s
3) The interior portion of the object, containing a copper tube with an iron rod in the middle, is completely identical to those used to store important religious texts.
4) The iron rod did not protrude above the bitumen layer. There was no way to attach a conductor to the iron rod.
5) The only people claiming to have built such a battery and successfully electroplated something with it failed to even document the purported experiments. Mythbusters built one, you can get almost a volt, but being powerful enough for electroplating is another story.
6) If the ancients accidentally built a 1V battery it is unlikely they would ever have known that they had done so. The most obvious use would be as a heater. It would take a lot of experimentation over a long time to find other uses, and that wouldn’t happen unless it was a widespread technology.
7) There don’t appear to be any archaeologists who think it was a battery.
8) The object itself has two competing stories of how it was discovered. It was either dug up in an undocumented dig, or else found in the basement of the museum. The story was told both ways. That same person is the only source for the idea that it is a battery, and he also was mistaken about the nature of fire-gilding. This all fits together to tell a more realistic story than the one about a battery. If you thought the fire-gilding was electroplating, it would them make perfect sense to guess “battery” when you saw this object. But no. And no, thinking so doesn’t convert the idea into a valid hypothesis, much less a theory. It is just an idea that got thrown at the wall, bounced off, and stuck to a tabloid sitting nearby.
It is quite clearly a scroll placed in a pot that did not survive the test of time. There is no reason to believe that it was ever an electrochemical cell, or that it even contained an electrolyte during use. The acid residue is consistent with papyrus.
The battery idea doesn’t appear to be a scientific theory or hypothesis, but rather something written in books intended to entertain people.
That 0.6 Volts per cell potential is actually very useful.
How do you balance the cells in series? Very simple – connect a string of silicon diodes in parallel. When the cell fills up, it gets bypassed automatically.
That would require getting a bunch of exactly matched diodes, not sure how cost effective that is compared to a typical BMS. Technically you can already do that with any chemistry, just gotta chain more matched diodes together.
I think if that would work well it would already be used.
A “typical BMS” would do the same with at least 10x the number of components, so whatever the diodes cost you lose in the assembly
The reason it isn’t used is because most battery chemistries operate well above a volt, and that requires a zener type diode, and zeners aren’t as well defined because you’re operating in the backwards avalanche breakdown region – not to mention they can’t handle the bypass current.
The reason why the chaining doesn’t work well is because your tolerances stack up. 1% mismatch might be tolerable for a single cell, but if you stack four 1% tolerance diodes, worst case you’ll be 4% off and that no longer works for you, and the parts need to be made to a tighter tolerance at greater expense.
Hence why the scheme is only reasonable if you can use a single diode, but for most battery chemistries that requires using the diode in reverse in the zener region so you can get a higher breakdown voltage than the standard 0.5-0.7 Volts of a simple silicon rectifier diode.
Most solar lanterns for example use a zener diode to clamp the battery voltage down to prevent overcharging. It works as long as the charging current isn’t very high – otherwise you need to add an active load that is switched by the zener. Standard silicon diodes on the other hand are easily made to handle many amps of current.
Note that they still would dissipate the fill charging power of one cell. Maybe not a problem, but something to take into account.
and then one have to consider the effects of temperature on cell and diode voltage. Battery relies on chemistry and that is temperature dependent.
Indeed! Let’s assume the battery and diode start cold, and the battery is charged such that the diode is only barely conducting, and then the temperature rises because of external heat.
When the temperature rises, the voltage of the battery rises, while the junction voltage of the diode drops (silicium diodes have a negative temperature coefficient). Current starts to flow from the battery through the diode, heating the diode where most energy is dissipated. At the same time, the losses in the battery heat it slightly, and it is heated by being in close proximity to the hot diode. Battery voltage rises, diode voltage drops further, increasing the current.
This isn’t going to end well.
It’s not going to end up with a runaway because the diode voltage won’t drop arbitrarily low (it increases with current), and the battery cell won’t go arbitrarily high (and it starts to drop anyways).
If anything, the standby voltage of a typical battery should be kept lower at high temperature, so the diode fills that function as well by not allowing the battery to charge up so high when it’s hot.
NASA did some tests, I can’t find the PDF right now, but with li-ion when you have groups of 4 cells in parallel, and you put those in series, they had no problem with balancing, even with a bad cell.
Balancing is a bigger deal with capacitors than with batteries. Batteries have enough effective-series-resistance that they should make a decent voltage divider; until a cell fails. But having multiple cells in parallel will prevent the other good cells from seeing an over-voltage when a single cell is having problems, which is the problem with being out of balance. It is rare for a battery with an internal short to be fully-shorted, normally a shorted cell has high resistance on the short and so that group self-discharges early, but can still charge normally and operate with reduced capacity. So 4 in parallel with one bad is very similar during charging to 3 in parallel, and the voltages divide (close to) normally.
Anyone here who has used ferric chloride to etch PCBs knows that this is not one of the more friendly substances in existence. Try getting the stains out of something it’s touched…even glass! I hear you can sometimes sand a layer off linoleum floor to get rid of the brown spots.
I’m going to hand out an educated guess that this sort of thing will be true for any energy storing chemistry – it’s more a matter of “pick yer poison”.
On another tack, not all batteries have to be light or tiny. I’m using around 50kwh worth of lead acid on my solar homestead. It’s a small shed (8lx4wx4h) worth that is a couple truckloads to move. I don’t sweat the heavy metals…lead is the most recycled substance in “ever” and the infrastructure is all set up for that – recyclers pay me for the old ones when I change them out. I don’t care how big they are, or most of the time, how heavy. I replace them at very long intervals – if you don’t abuse them and get good ones, 10 year lifespan is easy, and some will go 25 years (warranty!)…No per-cell BMS is required, just fairly smart charge controllers. To get the life – you don’t deep cycle them often – mostly use around 10% a day worth of their capacity. It scales pretty linearly – use half as much, they last twice as long.
What’s the advantage to using half as much to last twice as long? The per unit cost remains the same doesn’t it? If it was use half as much to last three times as long I could see a benefit.
Your downtime, replacement time and labor etc aren’t free.
> Pick your poison
Or no nasty chemicals – here is the U.K. we have a big battery which only uses water. Look up Dinorwig. Only disadvantage is that it’s not very portable.
I got one near my backyard in Poland too, and you can ride scooter on them.
http://lh5.ggpht.com/-dYAxB7pnHiM/Tr2BR-fgf_I/AAAAAAAAB4U/NyeZVfvf6J0/Elektrownia%252520%2525C5%2525BCar%252520001a.jpg
That’s all dandy for places where you don’t have to go far to gain/lose a meter of elevation. Where I live it’s quite a distance. Sometimes the rainwater doesn’t know which direction to flow. :-)
We’vegot a non rechargable one over in Whalley Bridge too, only it’s been sat on the shelf too long and started to leak.
What’s the ALL-IRON BATTERY self discharge rate ? The Nickel–iron battery will last forever, but it’s self discharge rate is lousy.
Probably depends a lot on how good a job you do treating the cellulose paper with the sodium polyacrylate. It is not going to be perfect.
It apparently makes a difference which cellulose paper you choose, too. You want a smooth hard surface, but also high absorbency.
This is the weak part of the design, IMO.
Why the graphite anode? Can one not just use a sacrificial iron anode, just replace it if it wears out. Perhaps that will slowly alter the chemistry? I’m all for repairable cells if the replaceable parts are as basic as an iron plate.
Might be longevity issues too, but if you replace the right side (the side with no iron) with iron you’ve balanced the cell and the EMF is zero.
I was thinking it is probably conductive but not particularly reactive in this use, but I haven’t actually looked at the chemical reactions at all. My line of thinking was that it might be there to reduce the resistance of the iron plates.
The anode is iron, and it isn’t sacrificial because the cathode is also iron and the reaction is reversible.
The graphite sheets at the end are just current collectors. Like in a regular factory-built battery, the ends that you press against a conductor are not actually the cathode and anode but instead they’re uniform conductors with good surface area for connection. If you were building this to tight tolerances and installing it in a can you probably wouldn’t need that. But it is an easy way to build it in a loose pouch and still be able to get current out.
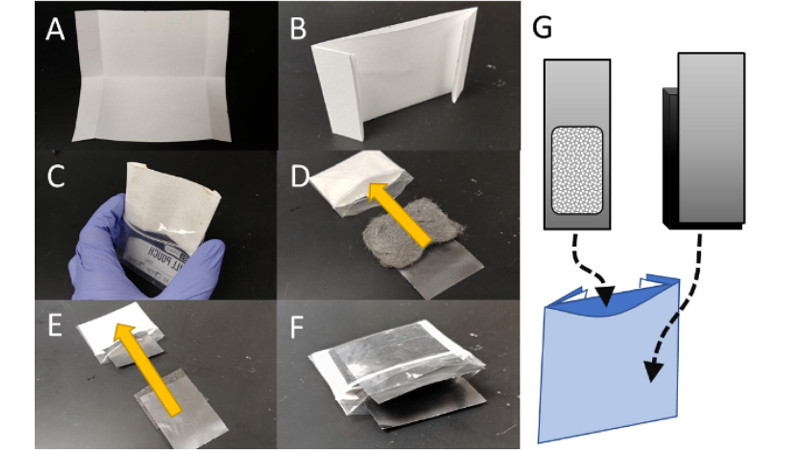
Since the voltage is so low, you’re going to want multiple cells in series, and the ones in the middle you can just use a spring clip (office supply type) to hold the graphite sheets in the middle together. (Pictured in the prototype, see pdf at link in story)
Many battery chemistries will work 600mAh, but the maximum discharge appears to only be a few mA.
– Only about 10 cycles are shown. However, if you look even closer at the 10 cycle “longevity” test, each charge and discharge cycle was only 1mA for 1 hour – so they have only shown that it has a 10-cycle life when storing 1mAh, which is considerably more expensive than any other rechargeable battery on the market.
If anyone here would like to seriously investigate this topic, the first thing to do really is to run an automated charge/discharge cycle for enough cycles to destroy the battery.
– In general, the reason to be concerned about this chemistry is that iron is extremely reactive. There is a note in the article about stability issues during cycle testing, and that the cell was not sealed. Sealing cells reliably can be difficult.
– Electrochemistry is black magic IMO (think RF for chemistry). There are a lot of side reactions that your basic chemistry classes taught you don’t happen, but they do. There may be other more complex, difficult to analyze issues to cause long (or short) term degradation.
– I would bet there are papers on iron batteries (probably not open access) from the last 100 years. Scientists have tried a whole lot of stuff.
I think that was addressed near the end of the paper – their test cells were made of kitchen steel wool and filter paper in a ziploc bag so aren’t optimised for power. There are changes they could make to reduce the internal resistance which would increase current capacity, but I wouldn’t expect a cell which I could assemble by hand to match the power of a commercial battery.
What about round-trip efficiency? If I remember that is problematic for edison cells, which end up at <50% overall.
If any of y’all are interested in another relatively non-toxic type of battery, check out ‘Vanadium Redox Batteries‘. A Canadian company has recently been commissioned to work on a 40MWh(!!!) flow battery project in China.
Boiled one side of aluminum foil. Also got 0.6 Volts. Similar number. Could their exist a common classification potential? Used to keep track of the changes. My guess. If create a battery with any ?changes you can tax it for procentage of energy.