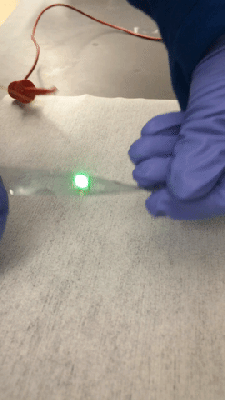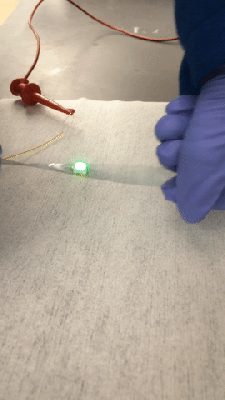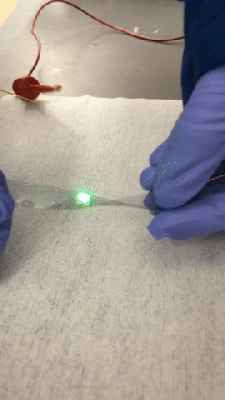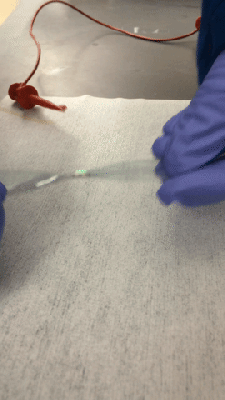
Fair warning: [Justin Atkin]’s video on how to make plasma, fusors, and magnetrons is a bit long. But it’s worth watching because he’s laying a foundation for a series of experiments with plasma, which looks like it will be a lot of fun.
After a nice primer on the physics of plasma, [Justin] goes into some detail about the basic tools of the trade: high voltage and high vacuum. A couple of scrap microwave oven transformers, a bridge rectifier, and a capacitor provide the 2000 volts DC output needed. It’s a workable setup, but we’ll take issue with the incredibly dangerous “scariac” autotransformer, popularized by [The King of Random]. It seems foolish to risk a painful death mixing water and line current when a 20-amp variac can be had for $100.
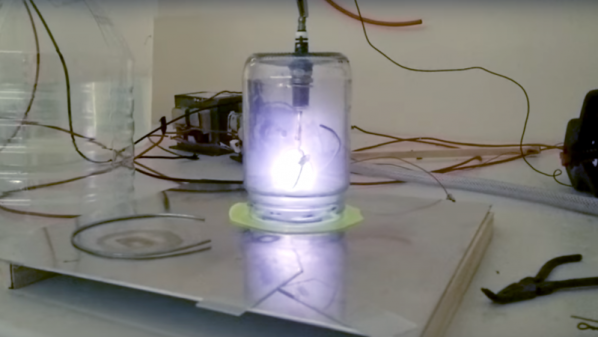
A decent vacuum pump will be needed too, of course; perhaps the money you can save by building your own Sprengel vacuum pump can be put toward the electrical budget. Vacuum chambers are cheap too — Mason jars with ground rims and holes drilled for accessories like spark plugs. Magnets mounted below one chamber formed a rudimentary magnetron, thankfully without the resonating cavities needed for producing microwaves. Another experiment attempted vapor deposition of titanium nitride.
It’s all pretty cool stuff, and we’re looking forward to more details and results. While we wait, feel free to check out the tons of plasma projects we’ve featured, from tiny plasma speakers to giant plasma tubes.
Continue reading “Put Plasma To Work With This Basic Toolkit”