In America, corn syrup is king, and real sugar hovers somewhere around prince status. We’re addicted to corn, and corn, in turn, is addicted to nitrogen. A long time ago, people figured out that by rotating crops, the soil will stay nutrient-rich, which helps to an extent by retaining nitrogen. Then we figured out how to make nitrogen fertilizer, and through its use we essentially doubled the average crop yield over the last hundred years or so.

Not all plants need extra nitrogen. Legumes like beans and soybeans are able to make their own. But corn definitely needs nitrogen. In the 1980s, the now-chief of agriculture for Mars, Inc. Howard-Yana Shapiro went to Mexico, corn capital of the world, looking for new kinds of corn. He found one in southern Mexico, in the Mixes District of Oaxaca. Not only was this corn taller than American corn by several feet, it somehow grew to these dizzying heights in terrible soil.
Shapiro thought the corn’s success might have something to do with the aerial, finger-like roots protruding from the cornstalk. Decades later, it turns out he was right. Researchers at UC Davis have proven that those aerial roots allow the plant to grab nitrogen out of the air through a symbiotic relationship with bacteria in that clear, syrupy mucus. The process is called nitrogen fixation.
Nitrogen Fixing is a Bit Broken
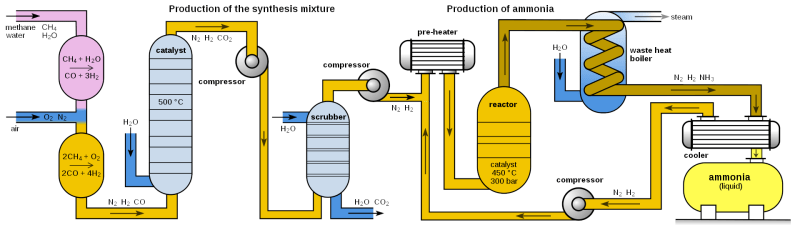
So if we already have nitrogen fertilizer, why even look for plants that do it themselves? The Haber-Bosch fertilizer-making process, which is an artificial form of nitrogen fixation, does make barren soil less of a factor. But that extra nitrogen in ammonia-based fertilizer tends to run off into nearby streams and lakes, making its use an environmental hazard. And the process of creating ammonia for fertilizer involves fossil fuels, uses a lot of energy, and produces greenhouse gases to boot. All in all, it’s a horrible thing to do to the environment for the sake of agriculture. But with so many people to feed, what else is there to do?
Over the last decade, the UC Davis researchers use DNA sequencing to determine that the mucus on the Sierra Mixe variety of the plant provides microbes to the corn, which give it both sugars to eat and a layer of protection from oxygen. They believe that the plants get 30-80% of their nitrogen this way. The researchers also proved that the microbes do in fact belong to nitrogen-fixing families and are similar to those found in legumes. Most impressively, they were able to transplant Sierra Mixe corn to both Davis, California and Madison, Wisconsin, and have it grow successfully, proving that the nitrogen-fixing trick isn’t limited to the corn’s home turf. Now they are working to identify the genes that produce the aerial roots.
One Step in a Longer Journey of Progress
We probably won’t be switching over to Sierra Mixe corn anytime soon, however. It takes eight months to mature, which is much too slow for American appetites used to a three-month maturation period. If we can figure out how to make other plants do their own nitrogen fixation, who knows how far we could go? It seems likely that more people would accept a superpower grafted from a corn cousin instead of trying to use CRISPR to grant self-nitrogen fixation, as studies have shown a distrust of genetically modified foods.
The issue of intellectual property rights could be a problem, but the researchers started on the right foot with the Mexican government by putting legal agreements in place that ensure the Sierra Mixe community benefits from research and possible commercialization. We can’t wait to see what they’re able to do. If they’re unable to transplant the power of self-fixation to other plants, then perhaps there’s hope for improving the Haber-Bosch process.












