Need some kind of battery for a project? You can always find a few Lithium-Ion (LiIon) batteries around! They’re in our phones, laptops, and a myriad other battery-powered things of all forms – as hackers, we will find ourselves working with them more and more. Lithium-Ion batteries are unmatched when it comes to energy capacity, ease of charging, and all the shapes and sizes you can get one in.
There’s also misconceptions about these batteries – bad advice floating around, fearmongering videos of devices ablaze, as well as mundane lack of understanding. Today, I’d like to provide a general overview of how to treat your LiIon batteries properly, making sure they serve you well long-term.
What’s A Battery? A Malleable Pile Of Cells
Lithium-Ion batteries are our friends. Now, there can’t be a proper friendship if you two don’t understand each other. Lithium-Ion batteries are tailored for human needs by the factory that produced them. As for us hackers, we’ll want to learn some things.
First thing to learn – a single LiIon “unit” is called a cell. An average laptop contains three or six Li-Ion cells, a phone will have one, a tablet will have from one to three. What we refer to as “battery” is typically one or multiple cells, together with protection circuitry, casing and a separate connector – most of the time all three of these, but not always. The typical voltage is 3.6 V or 3.7 V, with maximum voltage being 4.2 V – these are chemistry-defined, the same for most kinds of cells and almost always written on the cell.
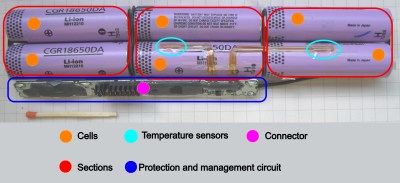
In multiple-cell batteries, the cells are arranged into a mixture of parallel and series arrangements, called a ‘configuration’. For instance, a typical 6-cell laptop battery could be described as a “3s2p” configuration, meaning “three sections in series, in which each section consists of two cells in parallel”. The voltage of such a battery is typically described as one of the battery voltages multiplied by number of series stages; a 3sXp battery voltage could be labelled as 10.8 V, 11.1 V, or 12.6 V.
Parallel cells can be functionally treated as a larger-capacity cell – for instance, if you have two 1000 mAh cells and you need 2000 mAh capacity, you can connect the two cells in parallel and get exactly what you need. Make sure not to parallel cells of significantly different capacity – then, one of those will take more charging current than it perhaps should. This arrangement has one problem: if one of the cells fails internally and starts self-discharging, it brings all the other parallel-connected healthy cells down as well. You might have encountered this failure mode if you have ever disassembled a dead laptop battery.
Shapes And Sizes, All The Same
There’s different physical form-factors for cells – cylindrical, pouch cells and prismatic. 18650 is the most popular cylindrical cell format, where 18 stands for diameter and 65 stands for length (in millimeters). These kind of cells, you’ll typically see in laptop and powertool batteries, electric transportation devices of all kinds.
 Pouch and prismatic cells are typically rectangular, with exceptions like cylinder-shaped cells in disposable vapes. These, you’ll see in phones, thinner laptops, tablets, and in general all kinds of handheld devices. I’ll refer to rectangular form-factor cells as “pouch cells” interchangeably.
Pouch and prismatic cells are typically rectangular, with exceptions like cylinder-shaped cells in disposable vapes. These, you’ll see in phones, thinner laptops, tablets, and in general all kinds of handheld devices. I’ll refer to rectangular form-factor cells as “pouch cells” interchangeably.
You’ll often see the term “LiPo” being used online when talking i.e. copter batteries, or just some pouch cells. LiPo technology is a subset of LiIon technology, typically indeed packaged into pouch cells, and is the same as LiIon for the purposes of this article. LiFePO4 is also a subtype of LiIon, it differs by some important aspects like voltages, but most of this article will apply still.
No matter which kind of LiIon cells you’re using, whether a 18650, some “LiPo” single-cell pack, or a pouch cell you took out of a dead smartphone, the electronic components required generally stay the same – mostly thanks to voltages involved being the same. For instance, if you have an ESP32 board with a JST-PH 2-pin input for a battery, it’ll function whether you have a 18650 in a holder with soldered wires, or a pouch cell you got from Adafruit. It isn’t arcane magic – you only have a few rules to follow. Here’s everything you’d ever want to know.
Bring Your Multimeter Out
 LiIon batteries don’t like being overdischarged; as a rule, you don’t want to get a LiIon battery below 2.5 V – 3.0 V, depending on how much current you’re trying to draw at that voltage. Below a certain voltage, LiIon batteries suffer irreversible changes and lose their capacity. 3.0 V is my personal threshold, making sure my batteries last even longer. No, it’s not a requirement that you add an ADC to your payload – just make sure your battery has a protection circuit; those typically are set to protect from 2.5 V overdischarge, and you can get 3.0 V ones too if you’d like.
LiIon batteries don’t like being overdischarged; as a rule, you don’t want to get a LiIon battery below 2.5 V – 3.0 V, depending on how much current you’re trying to draw at that voltage. Below a certain voltage, LiIon batteries suffer irreversible changes and lose their capacity. 3.0 V is my personal threshold, making sure my batteries last even longer. No, it’s not a requirement that you add an ADC to your payload – just make sure your battery has a protection circuit; those typically are set to protect from 2.5 V overdischarge, and you can get 3.0 V ones too if you’d like.
If you go gently on discharge, your battery will live longer. This is why your phone’s “0% battery” isn’t the real 0% of the battery’s capacity – there’s usually some juice left still, but using it up would have the battery capacity take a hit long-term. This is also why some laptops have a BIOS-accessible 80% battery charge level limitation for the purpose of extending the pack’s life expectancy – you trade some operational capacity for that, but it’s a worthwhile tradeoff to consider.

LiIon batteries want to be charged properly. You’ll see CC/CV mentioned – that refers to “constant current/constant voltage”, where first the battery is fed a constant current with the cell increasing its voltage as it charges, and once its voltage reaches some threshold, the charging mode is switched to keep the charging voltage somewhat constant and decrease the current over time instead. This two-stage charging process requires a charging IC – thankfully, those are omnipresent. If you have a device accepting a LiIon battery, it likely has an onboard charger; for your own devices, chargers are easy to find as modules.
When working with a small cells under 1000 mAh, you’ll want to ensure your charging current is half of the battery capacity number, or even less – so, for a 1000 mAh cell, it’d be 0.5 A or lower. For instance, the everpresent TP4056 modules are set up for 1 A current. Say, you tried to charge a 100 mAh battery with that – this is one of the rare cases where the cell might get fiercely dissatisfied with your treatment. Thankfully, for the blue “TP4056” modules, swapping a resistor is all it takes – and TP4056 boards often include a protection circuit as well!
Protection And Monitoring
Remember – LiIon cells can put a lot of current into whatever you connect to their terminals. When working with unprotected cells, it’s important that you don’t short them by accident. For instance, if you’d like to repair a powerbank, unsolder the negative terminal first – just like with car batteries. This will decrease the chances of you shorting the positive pin to to ground with your soldering iron – that would trip the protection circuit, cause a small spark, and possibly make your battery lose some of its capacity. This is one more reason why you have a protection circuit – these trip on overcurrent events.

Let’s recap: with a single-cell (or single-stage) battery, you’ll want a protection circuit, and there’ll be a charger somewhere in the picture. For LiIon packs where multiple cells are connected in series, you’ll want a protection circuit that’s wired into between-cell connections, for proper overdischarge monitoring – and you’ll also want some form of balancing wired into these, making sure that individual sections of the battery don’t overcharge. This protection+balancing circuitry combination is often referred to as “BMS” (battery management system).
If you end up having quite a few unused batteries or cells lying around, there’s something you could improve upon. It’s been found that you shouldn’t store your batteries long-term at 100% charge, as that might cause capacity loss – 50-60% charge is a better level for storage. So, if you want to put your cells on a shelf for a few months or more, it’s best if you get them to 3.8 V-3.6 V first. Not that full charge storage is going to kill your battery – can’t deny, I’ve been storing most of my cells fully charged and it hasn’t been a dealbreaker so far. This is a lot more of a concern for LiPo pouch batteries than LiIon cells.
Mechanical Manipulations

If you ever need to use blunt force to remove a glued-in cell, use non-sharp plastic tools instead of metal ones – and try to find a different way first. Let’s say, you want to free a pouch cell from an old tablet. First, try to squirt liberal amounts of isopropyl under the battery and let it soften, and twist the cell instead of bending it – you might find that you don’t need any tools at all. If you do need to pry the cell with something, use a cut up old credit card. Heating (i.e. hairdryer) is generally advised against.
One thing I strongly recommend you avoid is puncturing a puffed up pouch cell. Of course, sometimes a puncture happens by accident when refurbishing things, and you’re going to be fine if you move away from the cell for a bit, but don’t go around poking holes in spicy pillows for fun.
For 18650 (cylindrical) cells, it’s harder to puncture them, but still possible. One of these cases is taking a laptop battery apart, cutting through the battery case plastic with sharp metal tools – beware! The case is the negative terminal, so if you nick the shrink wrap, don’t make contact with the positive tip. However, if your 18650 plastic covering is missing or torn, that’s fine – it’s just a special kind of heatshrink, you can buy it for cheap and put it on the cell with hot air.

If you want to use 18650s in your own creations, you’re best off stocking up on holders, or printing some. The second best thing is spot welding metal strips onto them – some shops will do that for you, – and soldering wires to these. Soldering directly to 18650 cells is a tricky topic. The consensus is that it can be done, but it’s best if you don’t.
Applying heat to 18650 cell casing is best done in small amounts – as such, using a higher-power soldering iron for short amounts of time is probably the safest way you can do it. Perhaps one of your friends or a nearby hackerspace has a spot welder that you can use instead – or you can build your own?
Storing cells and batteries long-term isn’t rocket science – a free shelf will do. Make sure that they’re not mechanically at risk, aka they won’t fall, nothing heavy/sharp is on them, yadda yadda. For pouch cells without protection circuits, you’ll want to wrap the exposed terminals in something like kapton tape while they’re being stored, and put them somehwere fire-safe just in case. For 18650s, there’s cheap plastic cases you can store them in. Ammo boxes might be tempting, but make sure the metal doesn’t short the cells out as they rattle inside.
Be Picky When Making Friends
As hackers, we can get LiIon cells from a myriad of different sources. This is wonderful, since our projects come in all shapes and sizes – some will be fine with 18650, some can be powered by a smartphone battery or an ultrabook-scavenged flat cell, some are small enough that perhaps a vape battery will be a better bet.

Not all of these cells will be good for you, however – there’s some easy indicators that the cell is subpar and perhaps internally damaged, and these signs, you should watch out for. I’ll give you an acronym – L.U.S.H., which stands for “Lacerated, Undervolted, Subpar, Heated”.
Is the silver wrapper of your pouch cell cut? Is your 18650 dented on its side? Did you mishandle an RC car LiPo pack and now it’s squished together in the way it wasn’t before? If so, recycle it rather than reusing it. It’s okay to have a bit of a dent on your 18650’s negative terminal as you use tin snips to get rid of spot-welded strip remains. Other than that, there’s typically not a lot of mechanical leeway between the side wall of the cell and its internal layers of electrodes, and squeezing these layers together is a possible failure point.
If the cell is lower than 2.5 V, bin it. In case your cell has a protection circuit and that circuit’s output is at 0 V, you might want to measure before the protection circuit, directly on the cell’s contacts – typically gently cutting through kapton or plastic tape where necessary, perhaps using sewing needles held against your multimeter probes. Again, if below 2.5 V, bin it – and consider keeping the protection circuit, it might prove useful later! Also, be reasonable – if you’ve disassembled a laptop battery, where two sections are at 3.5 V and one is at 3 V, you better bin the latter.

Capacity loss means the cell has been damaged, and you don’t want to be using a damaged LiIon cell. For instance laptop battery makers use indicators of cell damage (overdischarge, overcharge and overheating) as a trigger to shut the battery controller down and brick the entire battery – and it makes sense, since they don’t want to have the liability. Even then, overall severe capacity loss isn’t usually considered a trigger for such protection. That said – if a battery has subpar capacity for its size or rating, bin it.
When charging the battery for the first time after it’s freed up from its previous host, make sure it doesn’t heat up noticeably – if it does, bin it. After charging, measure the voltage and write it down. It’s going to gradually decrease after you stop charging, but it shouldn’t drop too much. If it drops more than 0.3 V from where it was after you stopped charging, or just keeps dropping and dropping over the week, bin it – high levels of self-discharge typically go hand in hand with capacity loss.
Wait, What About Those Fires?
The risks of fire with LiIon cell batteries in typical hacker usecases are negligible. After all, we typically have thousands of LiIon cells in our general vicinity – consider all the phones, laptops, electric cars and the like, and they work wonders long-term across thousands of cycles. Typical LiIon fires happen because of mechanical damage to the cell, severe neglect of its electrical requirements, and rarely, mis-manufacturing. Having learned to treat your cells well and bin them when called for, you’re well-equipped to avoid battery fires ever happening. Want to learn more about LiIon battery fire safety specifically? Our colleague has talked about that in depth.
 When you need to dispose of a dead or subpar LiIon battery, are you understandably worried about its terminals getting shorted or the cell getting punctured after you bin it? Good news – a fully discharged battery is completely safe from any accidents, no longer having internal energy for combustion.
When you need to dispose of a dead or subpar LiIon battery, are you understandably worried about its terminals getting shorted or the cell getting punctured after you bin it? Good news – a fully discharged battery is completely safe from any accidents, no longer having internal energy for combustion.
How to fully discharge a cell? First, you could short the cell’s terminals with something like a 10 K through-hole resistor and leave it to discharge fully. Make sure to connect to the cell and not the protection circuit output! Another way I’ve seen people do is put the cell (or an entire pack) in saltwater and hold it there for a while – best done outdoors in an open container. This might sound a bit wack, but there’s scientific research aplenty! [Ed note: sounds wack! I just use a resistor.]
Go On, LiIon!
We haven’t always been well-equipped to handle LiIon batteries. It used to be that bargain bin devices used two diodes from a 5 V input to “charge” the battery – resulting in puffy and dissatisfied pouch cells. Nowadays, you’ll see hackers and makers equipping their devices with LiIon batteries left and right, protection boards and chargers are everpresent, and the skills ought to be ubiquitous too. Never stop learning, and you’ll always be equipped to make your projects more portable, failproof and trustworthy.
Next time, I’d like to talk about specifics – I want to give you a hacker-friendly cookbook with LiIon battery electronics examples that are easily repeatable at home. Until then – solder a JST-PH lead onto a smartphone cell and plug it into that ESP32 board you bought, see what that lets you achieve!















Nice article. I have a plethora of 18650 cells harvested from old laptop batteries. One tip — sometimes I pays to keep pairs together and to retain some of the metal strips and wires that were part of the laptop battery circuit. I will solder to wires and to the metal ribbons, but I personally avoid soldering directly to batteries. And yes, I like to use JST connectors to add to such for use in my projects.
Most commonly though I remove all the spot welded stuff and use them as 18650 cells in flashlights and such. I am continually amazed at how many perfectly useable cells are heading for the landfill. I just ran a set through a calibration cycle on my charger last night and they all yield 2000 maH — certainly quite useful to me.
Stackable Integrated Battery’s most popular products in Europe in 2022
URL: https://www.essvalley.com/stackable-integrated-battery
it’s when throwing the battery in the rubbish bin that i have the greatest fear of fire. i happened to read the advice to do cellphone battery transplant on a discharged battery, and i paid attention because i knew it was gonna be a pain (too much glue). sure enough, i pierced the battery while removing it but it was a non-event because it was flat.
so that’s the approach i’ve taken, i fully discharging the battery (ic clips, resistor, led, plus a month or more of sitting) before trashing it. i was nervous about it at first so i stabbed the bloated corpse the first time i tried this…and once it’s discharged, it seems pretty inert. no fireworks when the nail pierced all the layers.
i wish i knew a better way to dispose of them, still. even if there was a good battery recycling operation, i’d still have the fire worry about throwing a pile of compromised cells into someone’s cardboard box. if you don’t intentionally drain them then it can be really astonishing how much power they still have after sitting years.
yes.. that concerns me too, which is why I’ve added a section on discharge. That said, a battery that’s sufficiently discharged, won’t actually have all that much energy anymore, and most of the cells disposed of are low-capacity-left cells anyway. Also, hmm, a month shouldn’t be necessary with a low-value enough resistor – can always check the voltage with a multimeter, it’ll probably get very close to zero quite quickly!
yeah it can discharge a lot faster :) i just used the resistor+LED that was already in my hand…if i had more than one dying battery at a time i’d invent a better load. i like that the LED eventually gets so dim you can’t see it even with the lights out, really tells you where you stand
“LiIon batteries don’t like being overdischarged; as a rule, you don’t want to get a LiIon battery below 2.5 V – 3.0 V”.
Two Samsung J3 Luna batteries + INTELLIGENT CHARGER received from Amazon.
Batteries arrived charged to millivolts. :(?
intelligent charger charged both. :)
Intelligent charger starts with voltage 5.5-, them decreases max battery charge voltage.
My ‘stupid’ similar chargers start with voltage slightly above battery discharged voltage, then TRY to charge to max voltage.
Could it not be that the batteries needed activated by the charger before they worked, as in they didn’t output any voltage until their first charge. I’ve seen that a few times with BMSs.
In the US, Lowes has battery recycling bins. Home Depot probably does it, too. Check at the returns desk. Best to save all your cells up and recycle them
why we don’t have proper recycling procedures for all of this toxic bs is beyond me.
oh wait. no it’s not. money.
it would help if we had REAL right to repair. not this placate the politicians bullcrap.
Pretty sure you can drop them off at one of the 1000’s or retail places that recycle them.
Any professional advice regarding temperature? Why are there different hot and cold limits while charging, discharging, and storing them?
This isn’t so much the why, I guess that can be lumped into “because risks”. JEITA compliance is often cited. A bit too hot or a bit too cold then reduce charge current(usually 10c and 40c) usually to about half, way too hot or too cold, no charge at all. Usually 0 and 45/50C.
If it’s too hot reduce charge voltage by 0.05v, and if even hotter go down another 0.05v.
Also TIMERS!!!! I left 3 genuine gopro cells in a 3rd party charger thinking it’s like all the circuits I used. It was not. No temperature sensing going on and no timer disconnect. It just kept trying to hold it to 4.35 (these are LIHV cells) and they ballooned. Opened them and 3 super cheap common chargers that you find on a lot of those boards on alixpress — and guess what, no timers. This = dangerous if left plugged in.
Many of the bigger companies like TI and Maxim have great compliance and smarts built into their charger IC’s but they usually cost 2 – 3 times the el cheapo’s you can find.
https://www.ti.com/lit/an/slyt365/slyt365.pdf?ts=1662457386875
Great appnote on the temperature – it actually explains why and how temperature monitoring is used! Thing is, for hobbyists, temperature monitoring is typically not accessible – and I would argue, it’s not required, in my experience. If you notice a cell heat up noticeably (especially to 60C!), you bin it, that’s the basic recommendation. Furthermore, my advice is to charge at 0.5C already, which should stress the cell less in the first place.
Basically, decent cells don’t heat up during charging and discharging. This advice seems to be more for monolithic devices, where you can’t expect a user to be able to interact with the cell – the manufacturer sells the device, and then no longer has physical control over it, for years if not a decade. For hobbyists, our cells are usually accessible and we’re able to measure them, touch them, swap them. Developing a monolithic product for 1000pcs mfg with all its randomness, which has to work for years and never be accessible – yeah, I’d look into temperature control too!
Timers aren’t used for LiIon, because CC/CV measurements are reliable as an indicator of state of charge. They’re routinely used for some other kind of rechargeables (Ni-something? don’t recall) where state of charge isn’t easy to tell, but LiIon solutions never have a timer in them, because it’s usually just not needed. The charger itself shuts the charging current off.
As for the Gopro charger – I have no idea what happened there, and I’d like pictures of the board! TP4056 chargers are quite good – however, I’ve seen other, pretty subpar ICs being used in cheap products, especially in chargers meant to operate from a wall plug. Sadly, some manufacturers really want to pinch every penny they can, it’s like with those killer flashlights.
I’ve generally seen pouch cells puff up when they’re overvolted, or otherwise mishandled somehow. It’s not impossible that the ICs in your charger weren’t CC/CV ones, or perhaps were set for too high of a charging current, or somehow let a lot of ripple through if they were switching chargers. In fact, it’s not supposed to “try to hold it to 4.35” – it ought’ve shut off a few tenths of a volt before that.
Timers are the backup to proper charge termination which the ME4057’s inside didn’t do. If the cell is healthy enough then sure, but there are lots of situations where they might perform in spec but still not stop for some reason. Almost all charger IC’s developed in the last couple of years have safety timers now just for that reason. Stuck in pre-charge? Fast charge just never ending for some reason (wrong battery, cell damage and heating, etc) Better to shut that off and it seems the likes of most brands agree. Short list:
As for utility of timers, given that most companies that have engineered single cell chargers now include them such as below.
BQ25175 newly released, t_safety = 10 hour nominal in fast charge, 30 min in precharge (release 2021) – Same for BQ2517x
Different series BQ24232, 30 mins and 300mins
BQ2410x series, 10 hour safety timer
Nordic NPM1100, 7 hour fast charge cut off. (release 2020?)
MCP73833/4 Which harken from 2009 has safety timers. You can disable them, whereas new TI/Nordic/Maxim parts they can’t and in the few instances you can then you must intentionally over I2C on each and every power on for instance.
As for the Gopro, they are ME4057chips. I have two chargers both tested with dummy loads and they never cut off but they detected they where charged (LED status changed from charging to charged). Then they just sat there holding that voltage which is a no – no. They did not disconnect. Each charger had 3 IC’s, so 6/6 tested to never disconnect and from two very different looking batches.
The big difference is the reputable brans require full disconnection to reattempt. The ME4057s just try every 1.8ms…. over and over and over and over according to the datasheet.
The cold limit is to prevent lithium plating of the electrodes while charging, which would cause metal dendrites to grow and puncture the cell separator, resulting in a short circuit and a thermal runaway.
Lithium batteries can be charged all the way down to -25 C though if you keep the charging current very gentle, around 0.02C or less. Charging very slowly allows the lithium metal to dissolve back into the electrolyte and keep the cell from self-destructing.
The cold discharge limit is simply due to the cell internal resistance growing, so you get a greater voltage drop and the battery looks empty when it’s not.
I have a serious concern with the proliferation of lithium-ion batteries, and it’s not because I’m confounded by the chemistry, unaware of how to test them, or afraid of fire risks.
The concern is where do you get these batteries recycled? And if they’re not recyclable, why do we continue to build more and then subsequently “bin” them when they no longer perform, as if there is an unlimited supply of lithium?
I’m sick of throwing away batteries. Someone else will inherit this environment after I’m gone, and I don’t want to be a jerk and leave it a mess for them to clean up. If it can’t be recycled then the world doesn’t need it.
Sadly, recycling methods is not what I’m savvy about – we did do a few articles going into more detail on that, like this one or this one.
NiCd, NiMH and LiIon are all recyclable at most hardware stores. Jeers to Best Buy for no longer taking them. Alkaline recycling is pretty much unavailable in the USA (outside of CA, I assume).
They collect the batteries, but nobody really recycles them. They’re just disposed of as hazardous waste.
Depending on the battery chemistry (as some really are full of very toxic to something nasties – very much rarer these days though) throwing them away isn’t really bad environmentally – the content is generally just minerals in fairly common to nature forms packaged up a little more concentrated (at least before the can is damaged and the content starts disappearing). Though it is wasteful. It would be so much easier to recycle the content than mine ever more, which IS environmentally damaging. So if you can’t find a place to recycle them don’t feel too awful about binning them, as crimes against future generations go its almost meaningless compared to so much else in the day to day lives of most remotely developed nations…
That said in the UK at least the HWRC or ‘the tip’ I think I got the acyronm correct do take battery, and as far as I know they are then very safely disposed of if not recycled. Quite alot of supermarket seem to have battery recycling drop off points too – so I’d hope something similar exists in most developed nations. We have a little box in this house that fills up with ’em till either its enough to be worth a trip of its own or more likely it tags along on some required journey.
In Germany, all stores that sell LiIons / products are required to take them for recycling. So, like every hardware store.
But also your local recycling center. Or hazmat disposal place if you don’t have one.
It’s absolutely horrible that lithium batteries aren’t being recycled more.
Recycling batteries is kinda like recycling plastic bottles: they are collected, but not actually recycled except for some small experimental projects that run at a loss on public funding.
Back in the day when the soda companies actually recycled plastic soda bottles, they were made five times as thick to the point that you could use one for an ersatz baseball bat. Each bottle could go through the system 40-50 times, which meant that many of the soda bottles you had at the store were all scratched up and sketchy looking. The bottle would shrink slightly each time it was hot-washed, so they were discarded when the volume of the bottle became too small to contain the drink.
Lithium ion batteries are nice but if you live in Hawaiʻi, theyʻre unavailable due to shipping restrictions.
Honestly wonder. You have no cell phones or laptops with Li batteries?
They just have to come by boat instead of air. Depending on the size of the battery they can go by air too. I had to keep this in mind for a power meter I was designing for India, size the backup battery to what could be air freighted.
I see… Thank you!
Not unavailable, just more difficult to get. Air shipping is limited to certain capacity, but you can get them by boat. We have plenty of electric vehicles in the islands, including scooters and ebikes, all with larger than air permissable batteries.
Nice use of the ʻokina, btw.
The article was written in a cute way, thumbs up for that. 🙂👍
Thank you! LiIon batteries have been quite lovely to me as I’ve been creating portable devices, and I deeply appreciate the value they bring!
“Don’t run…. we are your friends!” [zaaap!]
One of the worst wastes of these cells is the single use E-Cigarette. When I see them tossed out, I pick them up and extract the cell for use in projects. Big Clive on Youtube has a good posting on them. Search for “big clive vape”.
I’m glad you liked what I wrote, and – I don’t believe you meant it this way, but this does read like a jab at our other writers! Please try not to do that; each of our writers is part of the equation and provides something unique. These unique sides might not be apparent for every reader, but they’re absolutely there and observable, with each writer bringing their own unique take on what they cover. Without every writer’s work in covering hacks day-to-day and upholding the common standards, we wouldn’t exist – it’s not fair to single one of us in a way that minimizes others!
Indeed! Though I too thought this well written, in quality above average for HAD. But I’d not change any of the other writers styles – its both interesting to have variety and can lead to better more expansive comments too.
Plus as somebody who doesn’t claim any great writing skill, and can no doubt be caught committing terrible sins against proper writing with awful spelling and probably strictly legal but less than clear grammar…
“under 2.5v bin it”
Have to disagree there. I have plenty of cells “recovered” from less than that which have useful, sometimes even considerable, capacities.
Big Clive on that subject…
https://www.youtube.com/watch?v=sRwoYJyjZNo
Also Diode Gone Wild…
https://www.youtube.com/watch?v=QdWy0PJyUCA
Couldn’t address it for a while – got busy and then fell ill, sadly. I’ve seen batteries revived from under 2.5V, and I’ve done it for cells I wanted to revive, but I don’t believe this to be good general-purpose advice. The aim of this writeup is “do this and you will be okay”, which “charge from under 2.5V” does not fit for me – I don’t believe I can tell you’re guaranteed to be okay if you let your cells get under 2.5V. It’s good that Big Clive was successful with those cells, and while the second video is quite surface-level, I guess 50% of original (already measly) capacity counts for something? Facing the randomness that’s out there though, I prefer to stick to a guideline backed by manufacturers’ datasheets and protection circuits, when it comes to prescribing what others should do on average. I don’t believe it to be much of a loss – given the variability of cells one might want to revive, a good general-purpose safety guideline like this has value. I think it matters that, if you’d like to recover a cell from under 2.5V, you have to give it a decent deal of thought and research, given that we know it’s not going to always work out. After all, non-deep-discharged cells are plentiful if you look.
Putting anything that produces electricity into salt water generates hydrogen and chlorine. They’re really small quantities, I played with electrolysis multiple times as a kid and it’s safe, but why when a resistor, or even better a small filament lamp rated for the battery voltage, would do the job nicely? The lamp would also work as an indicator of discharge.
Yep, exactly – “best done outdoors in an open container”! the reason – say, you disassemble a bunch of laptop packs, and end up with like 15 dead cells. You sure you want to carefully attach a resistor to each one of them, and wait for each of them to die? Chucking them all in a container is just, streamlined, which is why there’s research on this afaiu – for larger-scale full discharge.
it looks like the reason it’s been researched is that there are recycling/waste management organizations trying to deal with thousands of cells at a time, each with an unknown state. so “just chuck them in the fuming water tank” seems pretty attractive, and dealing with the fumes is just a part of business
Nice article and yes and hackers and engineers we do understand (mind you I have had a cell catch on fire trying to remove it from an iPhone) but people don’t understand or care and I have seen batteries damaged and catching on fire (Cordless drill used as a hammer anyone?)
I keep bad cells at the bottom of the garden in a metal box waiting for the day they can be sent somewhere to be recycled.
When discharging a battery made of multiple cells with a single resistor, there is risk of getting negative voltage over some of the cells. This is especially prone to happen if the battery has failed due to a single cell malfunction, as is quite common.
But I don’t know if negative voltage charging actually increases the risk of fire at all. It certainly damages the cell, but it is getting disposed anyway.
It dissolves the copper current collecting foil at the anode, which can lead to the structural failure of the cell while you’re discharging it and possibly a fire.
ok, many solutions and many ideas
every uses different size/old/new cell
WHY not charging , discarging separately?
what do you mean? could you tell us more about what your question is?
>a fully discharged battery is completely safe from any accidents, no longer having internal energy for combustion.
False.
Li-Ion electrolytes are flammable (the solvent is) and deep-discharging a lithium battery causes metallic lithium to form, which reacts with air and water. Do not puncture or crush lithium batteries even when completely discharged – they will remain a fire hazard regardless.
I was wondering why nobody commented on that, seems like very dangerous advice.
Better advice than leaving it charged, as all that stored energy can oh so easily provide the activation energy to set the whole thing off damn nearly instantly.
Also define ‘fully discharged’ – if its to the point the low voltage cutoff would kick in its probably still too charged to really be as accident proof as it can get, but discharged as deeply as remotely practical you are probably still a pretty long way from no potential energy stored anymore true 0V ‘fully’ discharged, and in the region where it is about as accident proof as it can get…
That wasn’t the advice: they’re saying it is completely safe from accidents which just isn’t true.
Stackable Integrated Battery’s most popular products in Europe in 2023
https://www.essvalley.com/All-In-One-Stackable-ESS-pd41147420.html
well, if what you say is true (sources for the “metallic lithium” claim would be good!), then I don’t recommend people puncture these batteries once deeply discharged! short-circuiting the terminals ought to be okay still, I assume.
It’s kinda how the battery works. Lithium ions are intercalated within a carbon lattice on the anode (-), and within a metal oxide lattice on the cathode (+) side of the battery, as well as being dissolved in the electrolyte. There’s more lithium than is necessary to operate the cell, because some of it will become unavailable locked up in other compounds with the side reactions of the cell as it ages.
When the battery is either over-discharged or over-charged, metallic lithium gets plated on either electrode surface because no more will fit within the structure of the electrode. This also happens when the battery is charged or discharged way too fast, or when the battery is too cold to operate. Once it appears, this metallic lithium also has a property to grow “whiskers” by a process of crystallization, very much like tin whiskers for pure tin solder, which the reason why we don’t build batteries with plain metallic lithium electrodes and why we have to faff around with the carbon and cobalt oxide etc. The lithium whiskers or dendrites grow like dandelions through asphalt and pierce the cell separator, causing the cell to short out.
The other harm of over-discharging the battery is when there’s no more lithium at the anode. The electrolyte starts to dissolve the copper current collector foil instead. This begins to happen unevenly across the cell somewhere around 2 Volts. The 2.5 Volt low-discharge limit is to account for this and the fact that the cell may be sitting empty on the shelf for a long time, so it needs some margin for further self-discharge. If you then try to recharge the cell again, the copper is deposited back unevenly and causes mechanical stress on the electrode and the cell separator, which may cause them to break and short out.
That said, it is possible to design a lithium cell that is not hurt by discharge to zero volts. It’s just that it’s not cost-effective to do so, and it would probably not have very favorable aging characteristics.
Besides, even if it’s not in a state of deep discharge, the lithium ions dissolved in the electrolyte will react with air and moisture, making heat, hydrogen gas, and lithium hydroxide which is very caustic. The electrolyte is usually a hydrocarbon based solvent that is highly flammable in air, which is what makes the big yellow flames burst out of a broken cell. It may also have things like Fluorine in it, which makes it toxic to breathe the smoke.
Point being, you should never puncture or crush a lithium battery no matter what you have done with it. It is never safe from accident. It contains chemicals that are basically self-igniting in contact with oxygen and water, and between themselves, and readily serve as fuel for combustion.
I’ve successfully brought back 18650s from 1v scavenged from many sources, including 10 year old laptop batteries. Put them in a smart charger, charged, discharged and then charged again measuring the mAh, written on the cell with a sharpie and if I need it I match it with a similar capacity one.
As an anecdote, I once had a 3 year old iPhone 5 that had swollen one summer in the heat. Took it apart and sure enough the battery pack had expanded around the middle. I couldn’t afford anything at the time, so I took it outside with a pin and gently poked a hole in the casing. Air escaped. No sign of smoke or anything dangerous but I left it out for about an hour just in case, put some electrical tape over the pinhole and popped it back in. Worked just fine. There was notably less capacity on it than the week before but it worked fine for another year before something else broke.
Stackable Integrated Battery’s most popular products in Europe in 2022
http://www.essvalley.com/stackable-integrated-battery
Stackable Integrated Battery’s most popular products in Europe in 2023
http://www.essvalley.com/stackable-integrated-battery
Stackable Integrated Battery’s most popular products in Europe in 2023
http://www.essvalley.com/stackable-integrated-battery