Under normal circumstances, if an electronic gadget in your pocket suddenly became hot to the touch, it would be cause for alarm. But not so with the Go Warmer. This lozenge shaped device is not only a USB power bank that can keep your mobile devices topped up, but is also doubles as a miniature heater that the manufacturer claims can bring its surface temperature up to 48 °C (120 °F) for several hours. You can hold in in your hand, put it in your pocket, maybe even sit on it if you’re particularly daring. The possibilities are endless, at least until the 4,000 mAh battery runs down.
For $14.99 USD, the Go Warmer certainly isn’t much of a deal when compared to other battery packs. Even if it does come with a swanky velveteen carrying pouch. But is it a good deal for one that can heat itself up without exploding? Let’s crack this metallic egg and find out.
Under the Skin
Since the whole point of this device is for it to get warm to the touch, I was glad to see they had the presence of mind to clad the Go Warmer with metal. But make no mistake, at just 0.5 mm thick, the two-part metallic outer shell isn’t structural. In fact, I’m sorry to say that there’s really no way to get inside the unit without doing unsightly damage to the easily bent plates and breaking off a few of the locking tabs.

Unfortunately, things don’t get much easier once you have the metal panels off. The red injection molded plastic frame is ridiculously strong, and the two sides are held together with eight clips that simply do not want to release. In the end I had to cut away some of the plastic to get the process going, and curse my way around the rest of the clips. After it finally popped open, I realized they’d even applied a liberal amount of glue for good measure. Seriously, if you don’t have an excellent reason to take one of these things apart, don’t.
Packed With Power
Poking and prodding at the frame of the Go Warmer was made all the more stressful by the assumption that on the other side of that bright red plastic was a lithium polymer pouch cell just looking for an excuse to blow up in my face. Each time a tool slipped, I was sure a spicy pillow was about to awaken on the workbench.
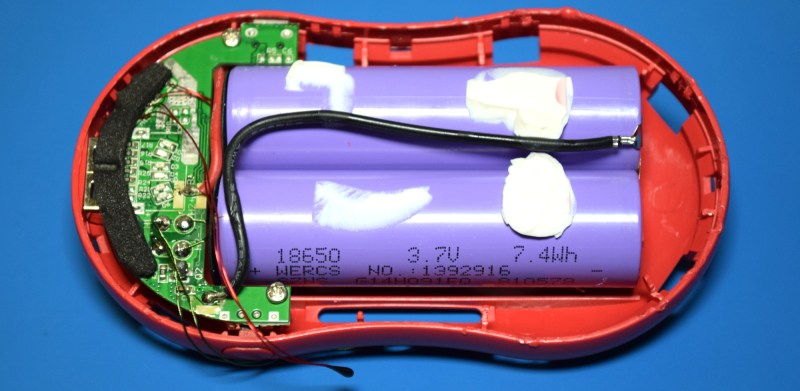
But thankfully, it turns out the Go Warmer is powered by a pair of 18560 cells put together into a neat little package. With proper insulation on the side of the cells and relatively beefy wires connecting them to the PCB, we might even allow ourselves to believe that whoever designed this little fellow actually knew what they were doing.
I was also pleased to see that the advertised 4,000 mAh figure appears to be legitimate, assuming the cells are halfway decent. To that end, forums are full of references to purple SZNS cells being a common sight in laptop battery packs and the like. Weighing in at the appropriate 45 grams per cell, I’m relatively sure these aren’t fakes and would potentially be worthy of salvage should you find a good deal on Go Warmers come Spring time. Though it does depend on how much work you’re willing to put into liberating them.
Two Brains in One Body
So we’ve got some metal side panels, a couple of little heating pads, and a pair of 18650s. Sure, wire those up with a simple switch and you’ll get something plenty hot. Thankfully, the Go Warmer is a bit smarter than that. Holding the button on the side cycles through the heater and power bank modes, and quick presses let you set the heat level. But how does it work?
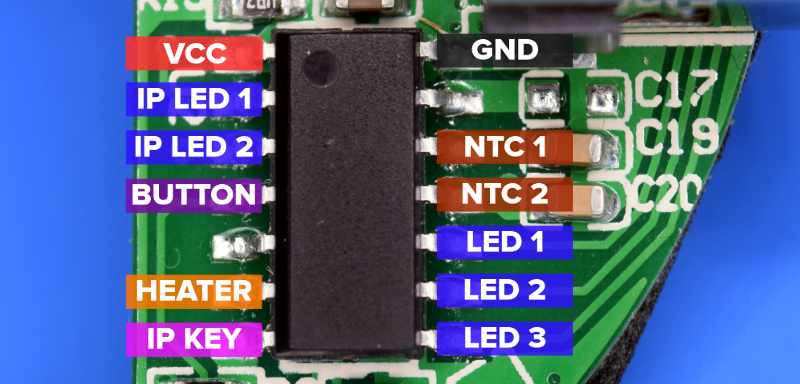
 Of the three chips on the PCB, only one has its markings intact: the IP5305 by Injoinic Technology. This is a complete power bank System-On-Chip that handles everything from driving status LEDs and the charging of the cells to the boost conversion up to 5 VDC.
Of the three chips on the PCB, only one has its markings intact: the IP5305 by Injoinic Technology. This is a complete power bank System-On-Chip that handles everything from driving status LEDs and the charging of the cells to the boost conversion up to 5 VDC.
As an all-in-one solution, the IP5305 only needs a single inductor and a handful of passives to get up and running. This seems like a handy enough chip that it might actually be worth salvaging from the board, though if you don’t mind waiting a month or two, they can be had for pennies on AliExpress.
But what about the two unmarked chips? We can surmise one is a voltage regulator based on the pin count and the fact it’s sitting between the battery and the rest of the board, so it stands to reason that the final 14-pin IC must be the microcontroller in charge of the heater aspect of the Go Warmer. After following some traces and a bit of buzzing with the multimeter, it wasn’t too difficult to figure out which pin was used for what.
There are a few interesting things to note here. Since the IP5305 was designed as a stand-alone device and has no provision for interfacing with other chips, the MCU is employing a bit of a hack by listening to two of its LED output pins to determine what the current battery and charge status is. Also note that the physical button on the Go Warmer is directly connected to the MCU, and the signal is only passed onto the KEY pin on the IP5305 when the software deems it necessary. This allows both chips to be controlled with a one button.
Even though both heaters are controlled simultaneously with a single pin connected to a MOSFET on the other side of the board, each one has its own independent NTC. Perhaps it’s taking an average temperature of both heaters, or maybe this is a safety feature so the power can be cut if one of the heaters starts getting hotter than expected. Snooping the sensor data and comparing that with the duty cycle of the MOSFET would give us a better idea of how everything is related, but I’ll leave that as an exercise for the particularly bored reader.
Style Versus Substance
From a practical standpoint, the Go Warmer is fairly lacking. With a capacity of just 4,000 mAh it’s not a very good power bank, and the tiny heating pads used to warm up the metal casing seem far too small. Even with the device cranked up to its maximum output, it only felt lukewarm to the touch. Arguably it would be better than nothing if you were out in the cold, but that’s hardly a rave review.
On the other hand, the internals ended up being far more interesting and well put together than I anticipated. The proper 18650 cells were a pleasant surprise, and the designers pulled off a rather clever hack by slipping their own MCU between the device’s physical controls and the off-the-shelf power bank SoC. Previous hybrid gadgets we’ve seen struggled to get their various functions working together in harmony, so the team behind the Go Warmer should be commended for executing it so well; even if the concept itself was a bit hokey to begin with.
















I admire the use of the word “hokey” in the last sentence. Nice write-up. And I’ll pass on getting one of these. (Not that I was feeling any overwhelming urge to start with).
For me, it was this paragraph that conjured up the most amusing mental image:
“but is also doubles as a miniature heater that the manufacturer claims can bring its surface temperature up to 48 °C (120 °F) for several hours.”
Is it too much for HaD editors to realize what a null statement that is and not post meaningless marketing propaganda to a tech focused site. I can match that claim with the energy available in a hearing-aid battery (I get to choose the test conditions). It means nothing about what this device really can do.
Its still useful to know what its supposed to do. How something is advertised is informative in its own right – if it claims to be a perpetual motion machine violating thermodynamics you know to avoid that company entirely on all products, as its not worth digging through anything else they may offer for the genuine stuff.
The fact it does turn out to be well put together, with some useful parts, but very mediocre in actual performance as the article goes on to say is enough for me afterwards…
Sure an actual temperature differential to ambient that it can reach would be nice – though under what conditions?
Personally the claim they make seems quite reasonable IFF you never let it go while its on, and its preheated in your pocket to near core body temp anyway eventually it should be able to get that warm, and because your holding it the ambient heat its effectively exposed to is whatever your surface body temperature is at the hands at that point, which will give it very little work to do staying at that warmer temperature (sounds positively horrid to me, who needs anything over 20, maybe 30C in your hands (unless it contains tea)??).
>who needs anything over 20
It has to stay a little bit warmer than your body temp in order to add heat to you. Otherwise it’s just slowing down how quickly your fingers are freezing. If that was the case, then you would be served just as well by bringing a warm rock with you and keeping it inside your jacket before you slip it in your mitten.
Aye, and I don’t know about you, but my fingers would be quite happy staying at 20, which if the ambient is really nasty would be a win – Numb/uncomfortable for my hands at least doesn’t happen for at least another 10C colder on their surface… So the self heating rock is useful as long as its above ambient temperature, as it stays warm on its own, and didn’t use body heat to warm in the first place, which reduces how hard you body has to heat itself.
Also if it is really cold enough out I’d not want something that hot – it would trick the blood flow in your hands to being high, and as soon as its put down/removed its going to be a much nastier shock, and cool you off faster as the surface blood flow there will be really high, because it thinks your trying to melt at 50C..
Can anyone please tell me what the two naked copper wires that are for? And how they need to be positioned when resembling
Is it too much for you to realize this isn’t a review of the product? The point is to take it apart and see what’s inside.
There are catalytic hand warmers that work on lighter fluid and operate up to 12 hours on a fill. They use little enough oxygen that you can put it in a fabric pouch, and that actually controls the rate of burning.
This is again one of those topics where vastly superior “old-school” methods are available, and trying to shoehorn a battery to do the job is just a terrible scam.
Actually this is incredibly old, with first commercial catalytic warmers appearing right after WW1 and the catalytic properties of platinum group metals being discovered even much earlier.
If you take a bunch of hemp/sisal/manila rope and rub it down to loose fibers, then rub some cooking oil (polyunsaturated oils: sunflower, canola, etc… Olive oil doesn’t work.) on it and leave it in a loose bunch, it will start to oxidize and warm up. Various other oils will heat up enough to catch fire.
I have heard tales of people starting fires in their garage by leaving rags saturated with teak oil lying about, after rubbing down their garden furniture.
And then there are the disposable pouch handwarmers that rely on the exothermic oxidation of fine iron particles (which I suspect were originally produced for the production of recording media). Oxidation has been going on for a *very* long time.
No word on warming yourself on a stack of 3.5″ floppies in your pocket though.
I’ve used those for over 30 years. Ye, they work great, but the smell of lighter fluid is a major downside. I recently bought a couple of these and I love them. Not only is there no smell, but no need to source or keep fluid on hand. These can be recharged in the wild with a solar panel. Normally, I am with you on old school versus modern, but in this case, I’ll go new school, just this once.
If one calculates the energy density of best 18650 cell (which has 3400 mAh at 48 grams) vs density of gasoline or white gas, it will get a factor of 50 to 1 in favor of much maligned fossil fuel. Yes, 48 grams of white gas or zippo fuel has 50 times more energy content than the best 18650 battery. That’s why I stick to catalytic hand warmers like Zippo, Peacock, etc for all my outdoor needs. These catalytic hand warmers can reach up to 70 C if left outside of their velvet pouch and get plenty of oxygen. I had used them in my mittens, socks when sleeping in the tent etc – with excellent results.
Of course those are not that spectacular to tear down and salvage parts …
They get hot enough that they work as radiation heaters, eg. when you’re working without gloves trying to fix something small. You just need something like a piece of space blanket or a disposable aluminum plate behind it to reflect the heat down at your hands.
Hmmm, interesting. I think we should see more teardowns here.
I like them too. Also, if you like teardowns, check out the Big Clive YouTube channel, he does a lot of interesting teardowns.
Yup, especially on days like today when Big Clive is on hiatus. This scratches that itch ;-)
Couldn’t you get the same effect by overcharging a Li-Ion battery?
Built into every cellphone.
Oooooh….
https://y.yarn.co/ed46dde9-b062-4f78-aa37-7c4f3e6601c2.mp4
I bought a couple of higher dollar versions ($25) of these a while back and I love them. I bought them to replace the lighter fluid hand warmers I’ve been using for 30 years and I am not disappointed. On the lowest setting, they get plenty hot and the highest setting is crazy hot. They work great. I still don’t know how they get so hot without damaging the cells. .
Derek
Would you mind telling me the brand and model, as I’m currently looking for hand warmers for my GF.
These types of hand warmers don’t really warm up unless they’re covered with something or in a pocket. They’ll always feel lukewarm in open air. Same goes for the iron oxide disposable warmers.
With all resistive electric heaters, the theoretical maximum temperature you can get them to is whatever temperature will destroy them. If you insulate them well enough, they’ll get hot no matter how little the heat output is! The 120 oF limit on these is likely set by the controls to limit damage if you insulate too well. There’s no reason they can’t hit that temperature, but it won’t be out in the open where losses are too high.
Temperature =/= heat output
Temperature = f(heat output, heat losses)
My Go Warmer unit has power. The light goes on when I turn on the light switch.
When I double click the operating button, a quick blink of a single blue light appears. When I single click or depress the operating button, a single quick blue light flashes and then for a second or two the two left red lights go on and then off.
What is happening? How do I fix it? Thank you.
Double click gives you the battery level in blue, long press turns it on and red lights give you power level. Once on, single click cycles through power settings. Long press turns it back off.
Thank you, “I-like-them.”
Yes, I understand how they operate. This one started that way. The second unit I have still works that way after the 3 months I’ve been using them on my winter walks.
However, the question I have here is about what I wrote and I copy: ‘When I double click the operating button, a quick blink of a single blue light appears. When I single click or depress the operating button, a single quick blue light flashes and then for a second or two the two left red lights go on and then off.’ The blue (even when charging) and red lights do not stay on and heat is not generated in the unit about which I’m writing.
Again, thanks for any tips you can provide.
I saw a video saying that’s the low charge warning.
Weird ! I had it on low l ft it on in my pocket and the sides of the warmer melted what would cause this?