Carbohydrate foams derived from dead trees are not the first material that springs to mind when considering building audio equipment. But really, there’s no reason not to explore new materials for jobs normally reserved for metal or plastic, and when pulled off right, as with this wooden ribbon microphone, the results can both look and sound great.
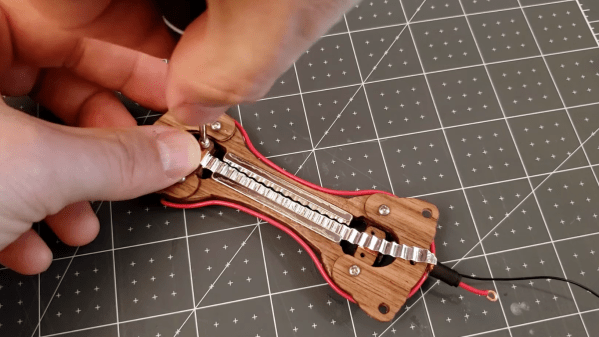
To be fair, there are plenty of non-wood components in [Frank Olson]’s replica of a classic RCA model 44 microphone. After all, it’s hard to get wood to exhibit the electromagnetic properties needed to turn acoustic energy into electric currents. But that doesn’t mean that wood, specifically walnut veneer, isn’t front and center in this design. [Frank] worked with thin sheets of veneer; cut into shape with a commercial vinyl cutter and stacked up with alternating grains, the wood was glued up with copious cyanoacrylate adhesive to form a plywood of sorts. The dogbone-shaped body was fitted with two neodymium magnets, leaving a gap just wide enough for the microphone’s ribbon diaphragm. That was made from a thin piece of aluminum foil that was corrugated using a DIY crimp roller. Suspended between the magnets and connected to leads, the mic element was adorned with a wood and fabric windscreen and suspended from elastic bands in a temporary frame for testing. The narration on the video below was recorded with the mic, which sounds quite nice to our ears.
We’ve seen ribbon microphones before, as well as wooden microphones, but this is the first time we’ve seen a wooden ribbon microphone. It looks as though [Frank] has more work he wants to do to finish it off properly, and we eagerly await the finished product.
Continue reading “Wooden You Love To Build A Ribbon Microphone?”