Most people have at least a fuzzy idea of what DNA is. Ask about RNA, though, and unless you are talking to a biologist, you are likely to get even more handwaving. We hackers might have to reread our biology text books, though, since researchers have built logic gates using RNA.
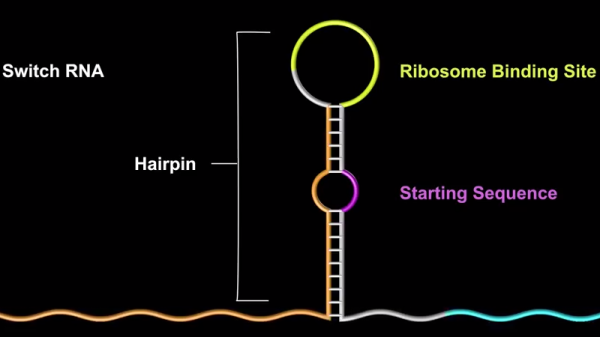
Sometimes we read these university press releases and realize that the result isn’t very practical. But in this case, the Arizona State University study shows how AND, OR, and NOT gates are possible and shows practical applications with four-input AND gates and six-input OR gates using living cells. The key is a construct known as an RNA toehold switch (see video below). Although this was worked out in 2012, this recent study shows how to apply it practically.
Continue reading “Synthetic Biology Creates Living Computers”