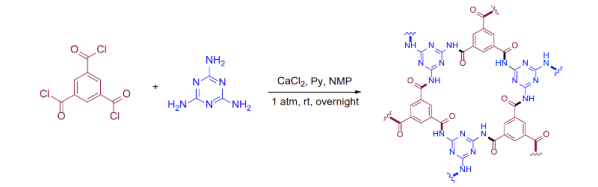
It seems like whenever the topic of rocket science comes up, the conversation quickly shifts to that of rocket fuels. As discussed in the excellent [Scott Manley] video below the break, there are many rocket fuels that can be found in some way, state, or form at your local grocery or liquor store. The video itself is a reaction to some college students in Utah who caused an evacuation when the rocket fuel they were cooking up exploded.
[Scott] himself theorizes that the fuel they were cooking was Rocket Candy, a volatile mix of sugar and potassium nitrate that is known to go Kaboom on occasion. And as it turns out, the combination might not even be legal in your area because as much as it can be used as rocket fuel, it can also be used for other things that go boom.
So, what else at your local megamart can be used to get to orbit? [Scott] talks about different kinds of alcohols, gasses, cleaners- all things that can be used as rocket fuel. He also talks about all of the solid reasons you don’t want to do this at home.
If this type of things gets your molecules excited, you might enjoy a bit we posted recently about using another grocery store staple to save Martian colonists from being held back by gravity.