Normally, videos over at The Signal Path channel on YouTube have a certain vibe, namely teardowns and deep dives into high-end test equipment for the microwave realm. And while we always love to see that kind of content, this hop into the world of cryogenics and liquid oxygen production shows that [Shahriar] has other interests, too.
Of course, to make liquid oxygen, one must first have oxygen. While it would be easy enough to get a tank of the stuff from a gas supplier, where’s the fun in that? So [Shahriar] started his quest with a cheap-ish off-the-shelf oxygen concentrator, one that uses the pressure-swing adsorption cycle we saw used to great effect with DIY O2 concentrators in the early days of the pandemic. Although analysis of the machine’s output revealed it wasn’t quite as capable as advertised, it still put out enough reasonably pure oxygen for the job at hand.
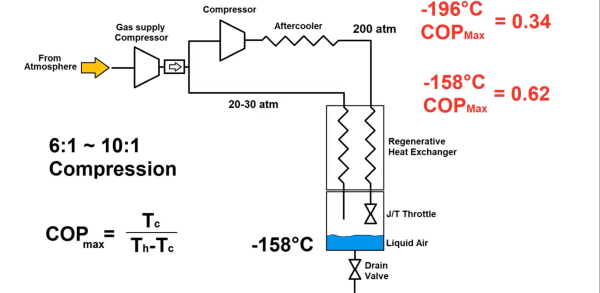
The next step in making liquid oxygen is cooling it, and for that job [Shahriar] turned to the cryocooler from a superconducting RF filter, a toy we’re keen to see more about in the future. For now, he was able to harvest the Stirling-cycle cryocooler and rig it up in a test stand with ample forced-air cooling for the heat rejection end and a manifold to supply a constant flow of oxygen from the concentrator. Strategically placed diodes were used to monitor the temperature at the cold end, a technique we can’t recall seeing before. Once powered up, the cryocooler got down to the 77 Kelvin range quite quickly, and within an hour, [Shahriar] had at least a hundred milliliters of lovely pale blue fluid that passed all the usual tests.
While we’ve seen a few attempts to make liquid nitrogen before, this might be the first time we’ve seen anyone make liquid oxygen. Hats off to [Shahriar] for the effort.
Continue reading “Making Liquid Oxygen: Far From Easy But Worth The Effort”