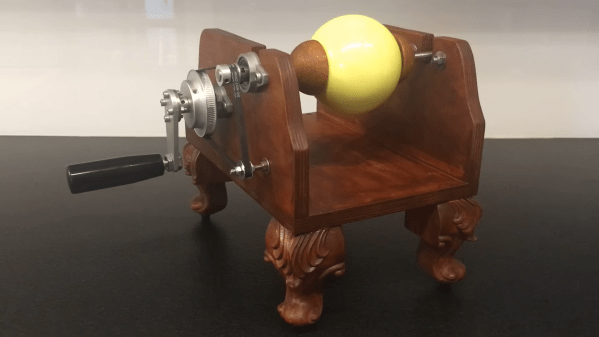
Although the basic concept of electrostatic attraction has been known since ancient times, it was only in the 17th century that scientists began to systematically investigate electrostatics. One of the first to explore this new field was Otto von Guericke, who constructed an electrostatic generator to help with his experiments. [Markus Bindhammer] has reconstructed this machine, which formed the basis for later work by the likes of Wimshurst and Van de Graaff. [Markus] kept his machine in an almost period-correct fashion.
Von Guericke’s machine consists of a sulfur ball mounted on a spindle that allows it to be rotated and rubbed against a piece of cloth. By doing so, the ball gains a charge that can be used to attract small pieces of material. [Markus] built a neat wooden frame with faux-antique carved legs and installed a handle, a spindle, and a belt-drive system to rotate whatever’s mounted on the spindle at high speed.
 All of this is beautifully documented in [Markus]’s video, but by far the most interesting part of his project is the process of manufacturing the sulfur ball. If you’ve always wanted one, here’s how to make one: first, melt some pieces of pure sulfur in a round-bottom flask using an oil bath. Then, turn on your vacuum pump to remove any air or water vapor trapped inside the liquid. Once the liquid is nice and clear, let it cool down and solidify very slowly; the sulfur ball can then be released from its container by breaking the glass with a hammer.
All of this is beautifully documented in [Markus]’s video, but by far the most interesting part of his project is the process of manufacturing the sulfur ball. If you’ve always wanted one, here’s how to make one: first, melt some pieces of pure sulfur in a round-bottom flask using an oil bath. Then, turn on your vacuum pump to remove any air or water vapor trapped inside the liquid. Once the liquid is nice and clear, let it cool down and solidify very slowly; the sulfur ball can then be released from its container by breaking the glass with a hammer.
While it sounds simple, we can imagine it took a bit of experimenting to get all those steps just right. The end result is a simple but useful machine to demonstrate basic electrostatics, which [Markus] is planning to use in science lectures. There are lots of interesting experiments you can do with static electricity, including building a basic motor.
Continue reading “Electrostatic Generator Project Starts With Molten Sulfur”