The 1970’s was the decade that illuminated the threat of acid rain to the citizens of the US. It had been known to exist several years before, but the sources of the problem did their best to suppress the information. It wasn’t until the environmental damage became significant enough to draw national attention that it would lead to the US enacting regulations to stop acid rain.
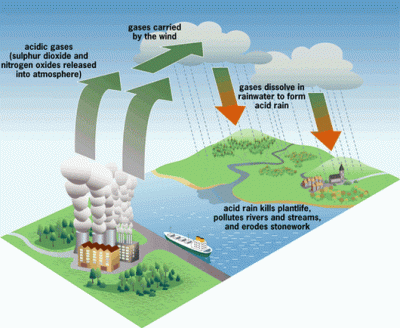
Truthfully, most of the public was probably still unaware of what acid rain actually was. The default mental image that comes to the mind of the non-chemist is large drops of battery acid raining down from the heavens and devouring everything. This is not quite the case, however. Pure water has a neutral pH of 7. Normal rain is actually slightly acidic as it picks up CO2 from the air, making carbonic acid. But when this “normal” rain mixes with the byproducts of industrial plants that pump out large amounts of SO2 (sulfuric dioxide) and NO (nitrogen oxide) into the atmosphere, it becomes even more acidic – down to a pH of 3. This “acid” rain has the acidity of citrus juice, so it’s not going to set the world on fire. But it will wreak havoc on local ecosystems.
The 1990’s brought with it tough government regulations on the output of SO2 and NO by large factories, pretty much eliminating acid rain in the US. The rise and fall of acid rain is a great example of why we should educate ourselves on the basic chemistries that define our lives, even though we might not be actual chemists. In this article, we’re going back to your first year of college and hash out just what defines an acid and base. And solidify our understanding of the pH scale. It is essential for the future biohacker to have this knowledge in their toolbox.