Being able to vocalize is one of the most essential elements of the human experience, with infants expected to start babbling their first words before they’re one year old, and much of their further life revolving around interacting with others using vocalizations involving varying degrees of vocabulary and fluency. This makes the impairment or loss of this ability difficult to devastating, as is the case with locked-in syndrome (LIS), amyotrophic lateral sclerosis (ALS) and similar conditions, where talking and vocalizing has or will become impossible.
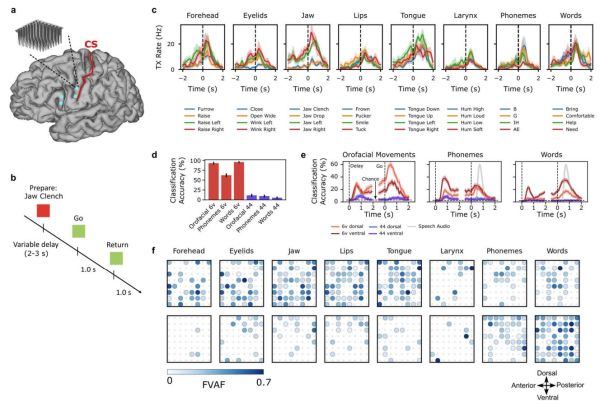
In a number of concurrent studies, the use of a brain-computer interface (BCI) is investigated to help patients suffering from LIS (Sean L. Metzger et al., 2023) and ALS (Francis R. Willett et al., 2023) to regain their speaking voice. Using the surgically implanted microelectrode arrays (Utah arrays) electrical impulses pertaining to the patient’s muscles involved in speaking are recorded and mapped to phonemes, which are the elements that make up speech. Each of these phonemes requires a specific configuration of the muscles of the vocal tract (e.g. lips, tongue, jaw and larynx), which can be measured with a fair degree of accuracy. Continue reading “Restoring A Person’s Voice Using A Brain-Computer Interface”