When a rainforest is clearcut for agricultural use, we only see the surface problems: fewer trees, destruction of plant and animal habitats, and countless other negative effects on the environment. A lurking problem, however, is that the soil is often non-ideal for farming. When the soil is exhausted, the farmers move further into the rainforest and repeat the process.
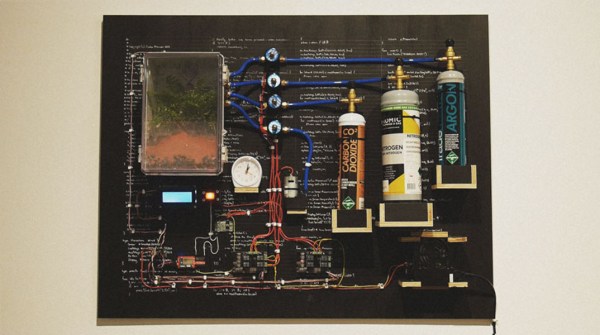
In the Amazon, however, there are pockets of man-made soil that are incredibly nutrient-dense. Figuring out how to make this soil, known as Terra Preta, on a massive scale would limit the amount of forest destruction by providing farmers a soil with more longevity which will, in turn, limit the encroachment on the rainforest. That’s the goal of this Hackaday Prize entry by [Leonardo Zuniga]: a pyrolysis chemical reactor that can make this soil by turning organic matter into a type of charcoal that can be incorporated into the soil to make Terra Preta.
As a bonus to making this nutrient-dense soil on a massive scale, this reactor also generates usable energy as a byproduct of processing organic waste, which goes several steps beyond simple soil enrichment. If successful and scalable, this project could result in more efficient farming techniques, greater yields, and, best of all, less damage to the environment and less impact on the rainforests.