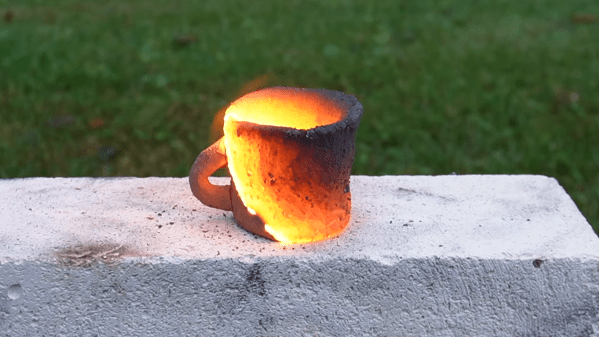Finely powdered aluminium can make almost anything more pyrotechnically interesting, from fireworks to machine shop cleanups – even ceramics, as [Degree of Freedom] discovered. He was experimenting with mixing aluminium powder with various other substances to see whether they could make a thermite-like combination, and found that he could shape a paste of aluminium powder and clay into a form, dry it, and ignite it. After burning, it left behind a hard ceramic material.
[Degree of Freedom] was naturally interested in the possibilities of self-firing clay, so he ran a series of experiments to optimize the composition, and found that a mixture of three parts of aluminium to five parts clay by volume worked best. However, he noticed that bubbles of hydrogen were forming under the surface of the clay, which could cause cracks during the firing. The aluminium was reacting with water to form the bubbles, somewhat like a unwanted form of aerated concrete, and for some reason the kaolinite in clay seemed to accelerate the reaction. Trying to passivate the aluminium by heating it in air or water didn’t prevent the reaction, but [Degree of Freedom] did find that clay extracted from the dirt in his back yard didn’t accelerate it as kaolinite did, and the mixture could dry out without forming bubbles.
This mixture wasn’t totally reliable, so to make it a bit more consistent [Degree of Freedom] added some iron oxide to accelerate the burn through an actual thermite reaction – some mixtures burned hot enough to start to melt the clay. After many tests, he found that sixteen parts clay, seven parts aluminium, and five parts iron oxide gave the best results. He fired two cups made of the mixture, a thin rod, and a cube, with mixed results. The clay expanded a bit during firing, which sometimes produced a rough finish, cracking, and fragility, but in some cases it was surprisingly strong.
The actual chemistry at work in the clay-aluminium mixtures is a bit obscure, but not all thermite reactions need to involve iron oxide, so there might have been some thermite component even in the earlier mixtures. If you need heat rather than ceramic, we’ve also seen a moldable thermite paste extruded from a 3D printer.
chemistry hacks646 Articles
Fail Of The Week: Beaker To Benchy More Bothersome Than Believed
Making nylon plastic from raw chemicals used to be a very common demo; depending where and when you grew up, you may well have done it in high school or even earlier. What’s not common is taking that nylon and doing something with it, like, say extruding it into filament to make a benchy. [Startup Chuck] shows us there might be a reason for that. (Video, embedded below.)
It starts out well enough: sebacoyl chloride and hexamethaline diamine mix up and do their polymerizing tango to make some nylon, just like we remember. (Some of us also got to play with mercury bare-handed; safety standards have changed and you’ll want to be very careful if you try this reaction at home). The string of nylon [Chuck] pulls from the beaker even looks a little bit like filament for a second, at least until it breaks and gets tossed into a blobby mess. We wonder if it would be possible to pull nylon directly into 1.75 mm filament with the proper technique, but quality control would be a big issue. Even if you could get a consistent diameter, there’d likely be too much solvent trapped inside to safely print.
Of course, melting the nylon with a blowtorch and trying to manually push the liquid through a die to create filament has its own quality control problems. That’s actually where this ends: no filament, and definitely no benchy. [Chuck] leaves the challenge open to anyone else who wants to take the crown. Perhaps one of you can show him how it’s done. We suspect it would be easiest to dry the homemade nylon and shred it into granules and only then extrude them, like was done with polypropylene in this mask-recycling project. Making filament from granules or pellets is something we’ve seen more than once over the years.
If you really want to make plastic from scratch, ordering monomers from Sigma-Aldrich might not cut it for ultimate bragging rights; other people are starting with pulling CO2 from the atmosphere.
Thanks to [Chaz] for the tip! Remember that the tips line isn’t just for your successes– anything interesting can find its home here.
Continue reading “Fail Of The Week: Beaker To Benchy More Bothersome Than Believed”
A Deep Dive Into Molten Bismuth
Bismuth is known for a few things: its low melting point, high density, and psychedelic hopper crystals. A literal deep-dive into any molten metal would be a terrible idea, regardless of low melting point, but [Electron Impressions]’s video on “Why Do Bismuth Crystals Look Like That” may be the most educational eight minutes posted to YouTube in the past week.
The whole video is worth a watch, but since spoilers are the point of these articles, we’ll let you in on the secret: it all comes down to Free Energy. No, not the perpetual motion scam sort of free energy, but the potential that is minimized in any chemical reaction. There’s potential energy to be had in crystal formation, after all, and nature is always (to the extent possible) going to minimize the amount left on the table.
In bismuth crystals– at least when you have a pot slowly cooling at standard temperature and pressure–that means instead of a large version of the rhombahedral crystal you might naively expect if you’ve tried growing salt or sugar crystals in beakers, you get the madman’s maze that actually emerges. The reason for this is that atoms are preferentially deposited onto the vertexes and edges of the growing crystal rather than the face. That tends to lead to more vertexes and edges until you get the fractal spirals that a good bismuth crystal is known for. (It’s not unlike the mechanism by which the dreaded tin whiskers grow, as a matter of fact.)
Bismuth isn’t actually special in this respect; indeed, nothing in this video would not apply to other metals, in the right conditions. It just so happens that “the right conditions” in terms of crystal growth and the cooling of the melt are trivial to achieve when melting Bismuth in a way that they aren’t when melting, say, Aluminum in the back yard. [Electron Impressions] doesn’t mention because he is laser-focused on Bismuth here, but hopper crystals of everything from table salt to gold have been produced in the lab. When cooling goes to quick, it’s “any port in a storm” and atoms slam into solid phase without a care for the crystal structure, and you get fine-grained, polycrystaline solids; when it goes slowly enough, the underlying crystal geometry can dominate. Hopper crystals exist in a weird and delightful middle ground that’s totally worth eight minutes to learn about.
Aside from being easy to grow into delightful crystals, bismuth can also be useful when desoldering, and, oddly enough, making the world’s fastest transistor.
Etching Atomically Fine Needle Points
[Vik Olliver] has been extending the lower resolution limits of 3D printers with the RepRapMicron project, which aims to print structures with a feature size of ten micrometers. A molten plastic extruder would be impractical at such small scales, even if a hobbyist could manufacture one small enough, so instead [Vik]’s working on a system that uses a very fine needle point to place tiny droplets of UV resin on a substrate. These points have to be sharper than anything readily available, so his latest experiments have focused on electrochemically etching his own needles.
The needles start with a fine wire, which a 3D-printed bracket holds hanging down into a beaker of electrolyte, where another electrode is located. By applying a few volts across the circuit, with the wire acting as an anode, electrochemical erosion eventually wears through the wire and it drops off, leaving an atomically sharp point. Titanium wire performs best, but Nichrome and stainless steel also work. Copper wire doesn’t work, and by extension, nor does the plated copper wire sometimes sold as “stainless steel” by sketchy online merchants.
The electrolyte was made from either a 5% sodium chloride solution or 1% nitric acid. The salt solution produced a very thin, fine point, but also produced a cloudy suspension of metal hydroxides around the wire, which made it hard to tell when the wire had broken off. The goal of nitric acid was to prevent hydroxide formation; it produced a shorter, blunter tip with a pitted shaft, but it simply etched the tip of the wire to a point, with the rest of the wire never dropping off. Some experimentation revealed that a mixture of the two electrolyte solutions struck a good balance which etched fine points like the pure salt solution, but also avoided cloudy precipitates.
If you’re interested in seeing more of the RepRapMicron, we’ve looked at a previous iteration which scribed a minuscule Jolly Wrencher in marker ink. On a more macro scale, we’ve also seen one 3D printer which used a similar resin deposition scheme.
Tube Furnace Is The Real Hotness
We aren’t sure what [theglassman] is working on, but based on his recent projects, we think it is probably something interesting. He’s been decapping ICs, growing oxide on silicon substrates, and has built a tube furnace capable of reaching 1200 °C.
What would you do with something that can melt cast iron? We aren’t sure, but maybe you’ll tell us in the comments. We do have a fair idea of what [theglassman] is doing, though.
The core of the oven is a quartz tube. Insulation is via refractory cement and alumina ceramic wool. The heating itself is classic Nichrome wire and a tiny thermocouple. The real key, though, is to the proper controller. [theglassman] suggests a ramp/soak controller. These allow you to program sequences that heat up and then stop, which, if done properly, can prevent your fragile quartz tube from cracking.
Blue Alchemist Promises Rocket Fuel From Moon Dust
Usually when an alchemist shows up promising to turn rocks into gold, you should run the other way. Sure, rocket fuel isn’t gold, but on the moon it’s worth more than its weight in the yellow stuff. So there would be reason to be skeptical if this “Blue Alchemist” was actually an alchemist, and not a chemical reactor under development by the Blue Origin corporation.
The chemistry in question is quite simple, really: take moon dust, which is rich in aluminum silicate minerals, and melt the stuff. Then it’s just a matter of electrolysis to split the elements, collecting the gaseous oxygen for use in your rockets. So: moon dust to air and metals, just add power. Lots and lots of power.
Melting rock takes a lot of temperature, and the molten rock doesn’t electrolyse quite as easily as the water we’re more familiar with splitting. Still, it’s very doable; this is how aluminum is produced on Earth, though notably not from the sorts of minerals you find in moon dust. Given the image accompanying the press release, perhaps on the moon the old expression will be modified to “make oxygen while the sun shines”.
Hackaday wasn’t around to write about it, but forward-looking researchers at NASA, expecting just such a chemical reactor to be developed someday, proposed an Aluminum/Liquid Oxygen slurry monopropellant rocket back in the 1990s.
That’s not likely to be flying any time soon, but of course even with the Methalox rockets in vogue these days, there are appreciable cost savings to leaving your oxygen and home. And we’re not biologists, but maybe Astronauts would like to breathe some of this oxygen stuff? We’ve heard it’s good for your health.
Unobtanium No More; Perhaps We Already Have All The Elements We Need
It’s been a trope of the news cycle over the past decade or so, that there’s some element which we all need but which someone else has the sole supply, and that’s a Bad Thing. It’s been variously lithium, or rare earth elements, and the someone else is usually China, which makes the perfect mix of ingredients for a good media scare story. Sometimes these things cross from the financial pages to the geopolitical stage, even at times being cited in bellicose language. But is there really a shortage?
The Colorado School of Mines say perhaps not, as they’ve released a paper from an American perspective pointing out that the USA already has everything it needs but perhaps doesn’t realize it. We’re surprised it seems to have passed unnoticed in a world preoccupied with such matters.
We’ve covered a few stories about mineral shortages ourselves, and some of them even point to the same conclusion reached by the School of Mines, that those mineral riches lie not in the mines of China but in the waste products closer to American industry. In particular they point to the tailings from existing mines, a waste product of which there is a huge quantity to hand, and which once stripped of the metal they were mined for still contain enough of the sought-after ones to more than satisfy need.
The history of mining from medieval lead miners processing Roman tailings to 19th century gold miners discovering that their tailings were silver ore and on to the present day, includes many similar stories. Perhaps the real story is economic both in the publicity side and the mining side, a good scare story sells papers, and it’s just cheaper to buy your molybdenum from China rather than make your own. We’ll keep you posted if we see news of a tailings bonanza in the Rockies.