A fundamental difficulty of working with nanoparticles is that your objects of study are too small for an optical microscope to resolve, and thus measuring their size can be quite a challenge. Of course, if you have a scanning electron microscope, measuring particle size is straightforward. But for less well-equipped labs, a dynamic light scattering system, such as [Etienne]’s OpenDLS, fits the bill.
Dynamic light scattering works by shining a laser beam into a suspension of fine particles, then using a light sensor to measure the intensity of light scattered onto a certain point. As the particles undergo Brownian motion, the intensity of the scattered light changes. Based on the speed with which the scattered light varies, it’s possible to calculate the speed of the moving particles, and thus their size.
The OpenDLS uses a 3D printed and laser-cut frame to hold a small laser diode, which shines into a cuvette, on the side of which is the light sensor. [Etienne] tried a few different options, including a photoresistor and a light sensor designed for Arduino, but eventually chose a photodiode with a two-stage transimpedance amplifier. An Arduino samples the data at 67 kHz, then sends it over serial to a host computer, which uses SciPy and NumPy to analyse the data. Unfortunately, we were about six years late in getting to this story, and the Python program is a bit out of date by now (it was written in Python 2). It shouldn’t, however, be too hard for a motivated hacker to update.
With a standard 188 nm polystyrene dispersion, the OpenDLS calculated a size of 167 nm. Such underestimation seemed to be a persistent issue, probably caused by light being scattered multiple times. More dilution of the suspension would help, but it would also make the signal harder to measure, and the system’s already running near the limits of the hardware.
This isn’t the only creative way to measure the size of small particles, nor even the only way to investigate small particles optically. Of course, if you do have an electron microscope, nanoparticles make a good test target.
nanoparticles9 Articles
DIY Calibration Target For Electron Microscopes
It’s a problem that few of us will ever face, but if you ever have to calibrate your scanning electron microscope, you’ll need a resolution target with a high contrast under an electron beam. This requires an extremely small pattern of alternating high and low-density materials, which [ProjectsInFlight] created in his latest video by depositing gold nanoparticles on a silicon slide.
[ProjectsInFlight]’s scanning electron microscope came from a lab that discarded it as nonfunctional, and as we’ve seen before, he’s since been getting it back into working condition. When it was new, it could magnify 200,000 times and resolve features of 5.5 nm, and a resolution target with a range of feature sizes would indicate how high a magnification the microscope could still reach. [ProjectsInFlight] could also use the target to make before-and-after comparisons for his repairs, and to properly adjust the electron beam.
Since it’s easy to get very flat silicon wafers, [ProjectsInFlight] settled on these as the low-density portion of the target, and deposited a range of sizes of gold nanoparticles onto them as the high-density portion. To make the nanoparticles, he started by dissolving a small sample of gold in aqua regia to make chloroauric acid, then reduced this back to gold nanoparticles using sodium citrate. This gave particles in the 50-100 nanometer range, but [ProjectsInFlight] also needed some larger particles. This proved troublesome for a while, until he learned that he needed to cool the reaction temperature solution to near freezing before making the nanoparticles.
Using these particles, [ProjectsInFlight] was able to tune the astigmatism settings on the microscope’s electron beam so that it could clearly resolve the larger particles, and just barely see the smaller particles – quite an achievement considering that they’re under 100 nanometers across!
Electron microscopes are still a pretty rare build, but not unheard-of. If you ever find one that’s broken, it could be a worthwhile investment.
Continue reading “DIY Calibration Target For Electron Microscopes”
Nanotechnology In Ancient Rome? There Is Evidence
Anything related to nanotechnology feels fairly modern, doesn’t it? Although Richard Feynman planted the seeds of the idea in 1959, the word itself didn’t really get formed until the 70s or 80s, depending on who you ask. But there is evidence that nanotechnology could have existed as far back as the 4th century in ancient Rome.
That evidence lies in this, the Lycurgus cup. It’s an example of dichroic glass — that is, glass that takes on a different color depending on the light source. In this case, the opaque green of front lighting gives way to glowing red when light is shining through it. The mythology that explains the scene varies a bit, but the main character is King Lycurgus, king of Edoni in Thrace.
So how does it work? The glass contains extremely small quantities of colloidal gold and silver — nanoparticles of gold to produce the red, and silver particles to make the milky green. The composition of the Lycurgus cup was puzzling until the 1990s, when small pieces of the same type of glass were discovered in ancient Roman ruins and analyzed. The particles in the Lycurgus cup are thought to be the size of one thousandth of a grain of table salt — substantial enough to reflect light without blocking it.
The question is, how much did the Romans know about what they were doing? Did they really have the means to grind these particles into dust and purposely infuse them, or could this dichroic glass have been produced purely by accident? Be sure to check out the videos after the break that discuss this fascinating piece of drinkware.
Continue reading “Nanotechnology In Ancient Rome? There Is Evidence”
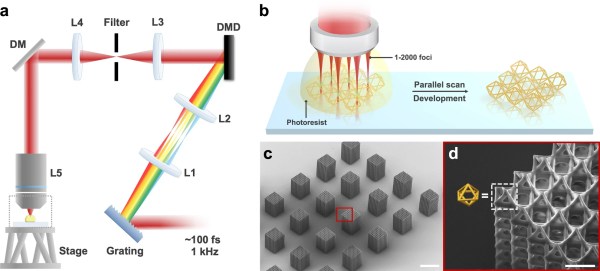
Holograms: The Future Of Speedy Nanoscale 3D Printing?
3D printing by painting with light beams on a vat of liquid plastic was once the stuff of science fiction, but now is very much science-fact. More than that, it’s consumer-level technology that we’re almost at the point of being blasé about. Scientists and engineers the world over have been quietly beavering away in their labs on the new hotness, nanoscale 3D printing with varying success. Recently IEESpectrum reports some promising work using holographic imaging to generate nanoscale structures at record speed.
Current stereolithography printers make use of UV laser scanned over the bottom of a vat of UV-sensitive liquid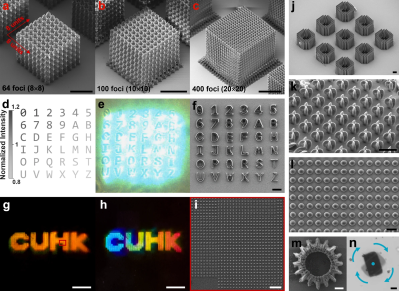 photopolymer resin, which is chemically tweaked to make it sensitive to the UV frequency photons. This is all fine, but as we know, this method is slow and can be of limited resolution, and has been largely superseded by LCD technology. Recent research has focussed on two-photon lithography, which uses a resin that is largely transparent to the wavelength of light concerned, but critically, can be polymerized with enough energy density (i.e. the method requires multiple photons to be simultaneously absorbed.) This is achieved by using pulsed-mode lasers to focus to a very tight point, giving the required huge energy density. This tight focus, plus the ability to pass the beam through the vat of liquid allows much tighter image resolution. But it is slow, painfully slow.
photopolymer resin, which is chemically tweaked to make it sensitive to the UV frequency photons. This is all fine, but as we know, this method is slow and can be of limited resolution, and has been largely superseded by LCD technology. Recent research has focussed on two-photon lithography, which uses a resin that is largely transparent to the wavelength of light concerned, but critically, can be polymerized with enough energy density (i.e. the method requires multiple photons to be simultaneously absorbed.) This is achieved by using pulsed-mode lasers to focus to a very tight point, giving the required huge energy density. This tight focus, plus the ability to pass the beam through the vat of liquid allows much tighter image resolution. But it is slow, painfully slow.
Continue reading “Holograms: The Future Of Speedy Nanoscale 3D Printing?”
Nanoparticles Rip Hydrogen From Water
Hydrogen fuel is promising, and while there’s plenty of hydrogen in the air and water, the problem is extracting it. Researchers have developed a way to use aluminum nanoparticles to rip hydrogen out of water with no additional energy input. It does, however, require gallium to enable the reaction. The reaction isn’t unknown (see the video below), but the new research has some interesting twists.
Aluminum, of course, is cheap and plentiful. Gallium, not so much, but the process allows recovery and reuse of the gallium, so that makes it more cost-effective. There is a patent pending for the process and — of course — the real trick is making the aluminum nanoparticles. But if you have that, this is a simple way to extract hydrogen from water with no extra energy and at room temperature. Since the reaction of creating aluminum oxide and releasing hydrogen with gallium is pretty well-known, it appears the real research here is determining the optimal properties of the aluminum and the ratio of aluminum to gallium.
While gallium isn’t a common item around the typical hacker’s workshop — unless you count the stuff bound up in semiconductors — it isn’t that expensive and it is relatively easy to handle. Hydrogen, though, not so much — so if you do decide to use this method to produce hydrogen, be careful!
We’ve seen gallium robots and even an antenna. So if you do get some of the liquid metal, there are plenty of experiments to try.
Growing Silver Nanoprisms With Light
Nanoparticles sound a bit like science fiction to minds of your average hacker — too esoteric and out of reach to be something we might get to work with in our own lairs — but [Ben Krasnow] of [Applied Science] over on YouTube has proven that they most definitely can be made by mere mortals, and importantly they can be tuned. With light. That’s right, nano particle growth appears to be affected very strongly by being illuminated with specific wavelengths, which locks-in their size, and thus defines their light-bending properties. This is the concept of photo mediated synthesis, which causes nanoparticles to clump together into different configurations depending on the wavelength. The idea is to start with a stock solution of Silver Nitrate, which is then reduced to form silver nanospheres which are then converted to larger silver nanoprisms, sized according to the wavelength of the illuminating source.
The process seems simple enough, with a solution of Silver Nitrate and Sodium Citrate being vacuum degassed to remove oxygen, and then purged by bubbling argon or nitrogen. Sodium Borohydride acts as a reducing agent, producing silver metal nanoparticles from the Silver Nitrate solution. The Sodium Citrate coats the silver nanoparticles, as they are produced, preventing them clumping together into a mushy precipitate. PVP (Polyvinylpyrrolidone) is added, acting as a colloiding agent preventing the coated nanoparticles from clumping together, and helping keep the solution stable long enough for the photo mediated synthesis process to complete. Finally, the pH is adjusted up to 11 using sodium hydroxide. The resulting silver nanoparticle stock solution has a pale yellow colour, and is ready for the final particle size selection using the light source.
(Polyvinylpyrrolidone) is added, acting as a colloiding agent preventing the coated nanoparticles from clumping together, and helping keep the solution stable long enough for the photo mediated synthesis process to complete. Finally, the pH is adjusted up to 11 using sodium hydroxide. The resulting silver nanoparticle stock solution has a pale yellow colour, and is ready for the final particle size selection using the light source.
The light source was custom made because [Ben] says he couldn’t find something suitable off the shelf. This is a simple design using a Teensy to drive an array of PAM2804 LED drivers, with each one of those driving its own medium power LED, one for each of the different wavelengths of interest. As [Ben] stresses, the naïve approach of trying to approximate a specific colour with an RGB LED setup would not work, as although the human eye perceives the colour, the actual wavelength peak will be totally wrong, and the reaction will not proceed as intended. The hardware design is available on MultiSpectLED GitHub for your viewing pleasure.
Nanoparticles have all kinds of weird and wonderful properties, such as making the unweldable, weldable, enabling aluminium to be 3D printed, and even enabling the production of one of our favourite liquid toys, ferrofluid.
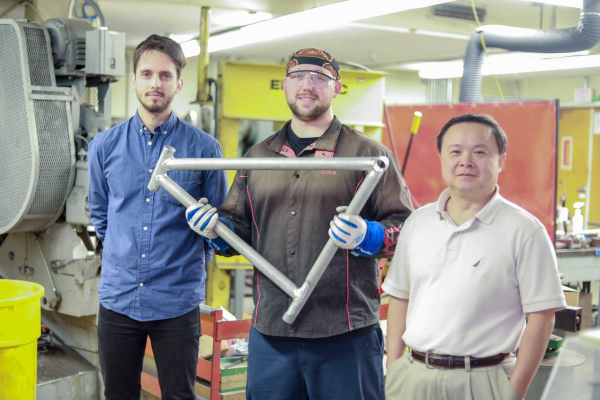
Nanoparticles Make Mega Difference For “Unweldable” Aluminum
Though much of it is hidden from view, welding is a vital part of society. It’s the glue that holds together the framework of the cars we drive, the buildings we occupy, the appliances we use, and the heavy machinery that keeps us moving forward. Every year, the tireless search continues for stronger and lighter materials to streamline our journey into the future of transportation and space exploration.
Some of these futuristic materials have been around for decades, but the technology needed to weld them lagged behind. A group of researchers at UCLA’s Samueli School of Engineering recently found the key to unlocking the weldability of aluminium alloy 7075, which was developed in the 1940s. By adding titanium carbide nanoparticles to the mix, they were able to create a bond that proved to be stronger than the pieces themselves.
Continue reading “Nanoparticles Make Mega Difference For “Unweldable” Aluminum”