In the late 1940s, the US Naval Research Laboratory used a few German-built V2 rockets to study cosmic rays from above Earth’s atmosphere. To do this, a nitrogen-powered cloud chamber was fitted inside the nose cone of these former missiles, sent aloft, and photographed every 25 seconds during flight. When [Markus] read about these experiments, he thought it would be an excellent way to study cosmic rays from a high altitude balloon and set about building his own Wilson cloud chamber.
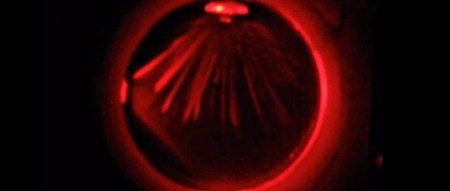
Cloud chambers work by supersaturating the atmosphere with water or alcohol vapor. This creates a smoky cloud inside the chamber, allowing for the visualization of radiation inside the cloud. Usually the clouds in these chambers are made in a very cold environment using dry ice, but rapidly decreasing the air pressure in the chamber will work just as well, as [Markus] discovered.
[Markus]’s small cloud chamber uses a CO2 cartridge to provide the pressure in the cloud chamber before dumping the CO2 out of the chamber with the help of a solenoid valve.
In the video after the break, [Markus] demonstrates his cloud chamber by illuminating the cloud with a laser pointer and introducing a few alpha particles with a sample of Americium 241. It looks very cool, and seems to be useful enough to count cosmic rays aboard a balloon or amateur rocket.
Continue reading “Researching Cosmic Rays With Cloud Chambers”