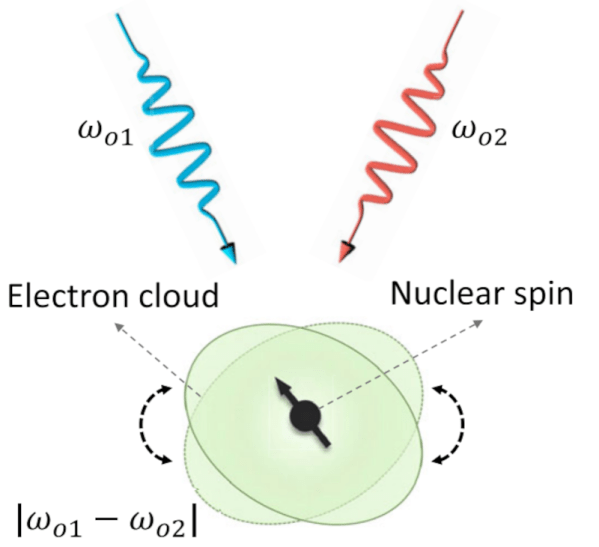
Quantum computers are coming, but there are still many problems with realizing practical machines. One is finding a reliable and affordable way to encode qubits — the basic unit for quantum computers. MIT researchers have a proposal. By using two slightly different colored lasers, they can manipulate nuclear spin. This isn’t the first time someone’s tried to use light to impact spin, but according to MIT, the other methods use an indirect coupling which is more prone to noise, something that limits the viability of quantum computers. They published a recent paper on the process if you want to read more.
Nuclear spin has weak interactions, but the new method doesn’t require intermediate steps, so it may be much more practical than previous methods. MIT mentions that typical quantum elements have coherence time limits, which means data stored in them becomes useless in less than a second. The new method promises to have coherence times measured in hours.
The method is known as the optonuclear quadrupolar effect or ONQ. From the paper:
[The ONQ effect] is second order in the electric field and nuclear spin I, as mediated by the quadrupole electric coupling, and is thus one of the nonlinear optical (NLO) responses of materials present in perfect crystals. Via the ONQ effect, nuclear spins can be coherently controlled by two-color photons, without electron spins as the media.
If you understood that, you should probably head over and read the rest of the paper. Meanwhile, the rest of us are waiting for our quantum Arduino.