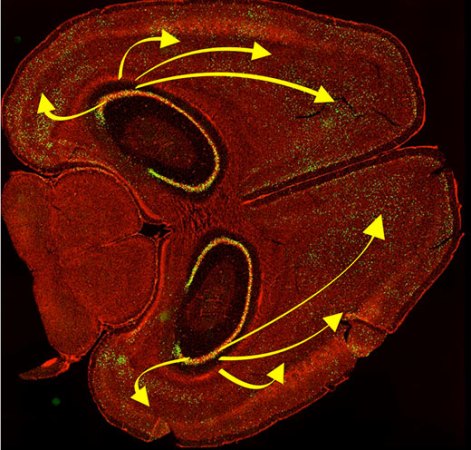
When auditory cells are modified to receive light, do you see sound, or hear light? To some trained gerbils at University Medical Center Göttingen, Germany under the care of [Tobias Moser], the question is moot. The gerbils were instructed to move to a different part of their cage when administrators played a sound, and when cochlear lights were activated on their modified cells, the gerbils obeyed their conditioning and went where they were supposed to go.
In the linked article, there is software which allows you to simulate what it is like to hear through a cochlear implant, or you can check out the video below the break which is not related to the article. Either way, improvements to the technology are welcome, and according to [Tobias]: “Optical stimulation may be the breakthrough to increase frequency resolution, and continue improving the cochlear implant”. The first cochlear implant was installed in 1964 so it has long history and a solid future.
This is not the only method for improving cochlear implants, and some don’t require any modified cells, but [Tobias] explained his reasoning. “I essentially took the harder route with optogenetics because it has a mechanism I understand,” and if that does not sound like so many hackers who reach for the tools they are familiar with, we don’t know what does. Revel in your Arduinos, 555 timers, transistors, or optogenetically modified cells, and know that your choice of tool is as powerful as the wielder.
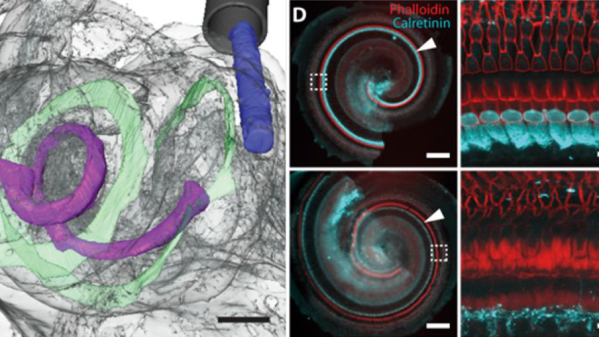
Optogenetics could become a hot ticket at bio maker spaces. We have talked about optogenetics in lab rodents before, but it also finds purchase in zebrafish and roundworm.



![The kind of travesty that can occur when [Stefan Kiese] doesn't have access to nice project boxes.](https://hackaday.com/wp-content/uploads/2016/07/img_3466.jpg?w=250)