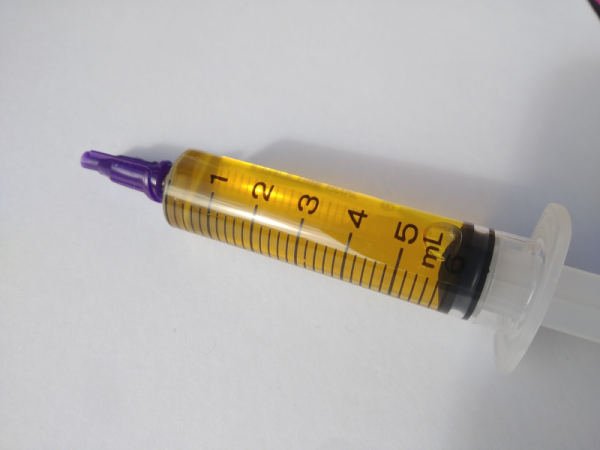
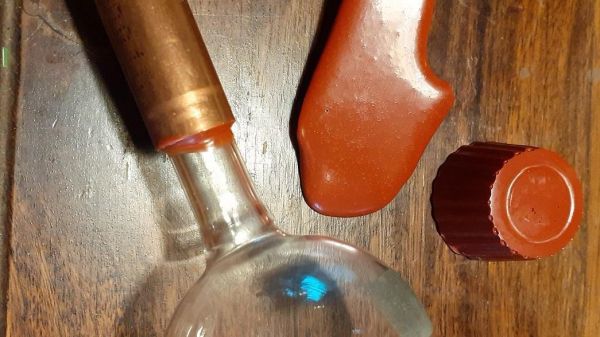Soldering flux is (or at least, should be) one of the ubiquitous features of any electronics bench. It serves the purpose of excluding oxygen from a solder joint as it solidifies, and in most cases its base is derived from pine rosin. Most of us just buy flux, but [pileofstuff] is having a go at making his own.
He starts with a block of rosin and a couple of different solvents. Isopropanol we’re happy with, but perhaps using methanol for something to be vaporized within breathing distance isn’t something we’d do. At about 25% rosin to solvent ratio the result is a yellow liquid flux, which he tests against some commercial fluxes. The result is a reasonable liquid flux, something which perhaps shouldn’t be too much of a surprise, and is a handy piece of information to store away should we ever be MacGuyver-like stuck in a pine forest with a need to save the day with electronics.
It would be interesting to try the same technique but with a solvent selected to soften the rosin for a paste flux, and perhaps any chemists among our readership could enlighten us about just what rosin is beside the heavy fractions left after extracting the volatiles from pine resin.
In the past we’ve taken a close look at how solder really works.